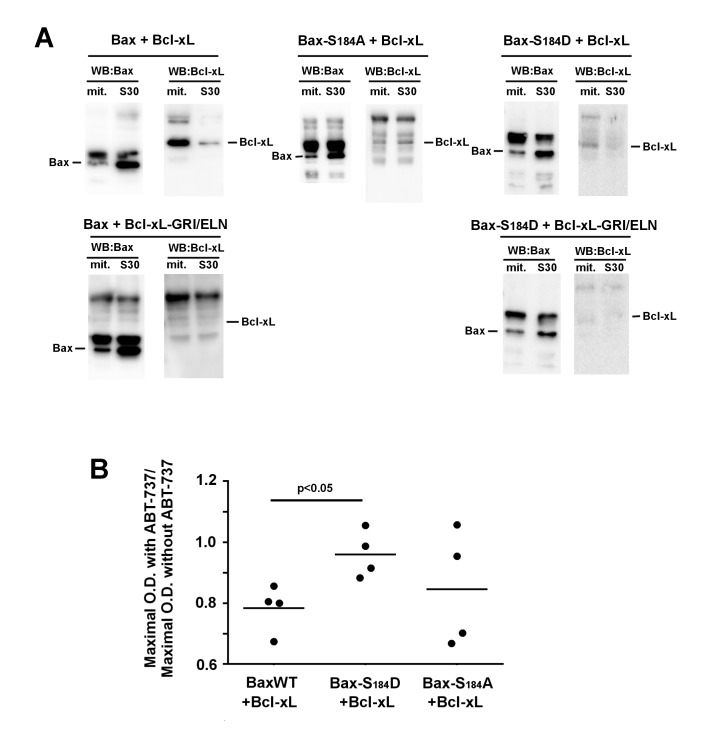
Figure 4. FIGURE 4: Interaction between Bax and Bcl-xL.
(A) Immunoprecipitation experiments were done on mitochondria and S30 fractions from cells expressing indicated Bax mutants with or without Bcl-xL or Bcl-xL GRI/ELN, as a negative control. 2 mg proteins of each fraction were immunoprecipitated with an anti-Bax antibody (2D2, Sigma). Blots are representative of experiments that have been done 5 times for Bax/Bcl-xL, 3 times for Bax-S184A/Bcl-xL and Bax-S184D/Bcl-xL, and twice for negative controls with Bcl-xL-GRI/ELN. Inputs were omitted for clarity and can be seen in Fig. S1. (Left) (Top) The IP against Bax showed the interaction with Bcl-xL only in the mitochondrial fraction. (Left) (Bottom) and (Right) (Bottom) The absence of signal when BaxWT or Bax-S184D were co-expressed with Bcl-xL GRI/ELN evidenced the specificity of the signal. (Middle) Bax-S184A was co-expressed with wild-type Bcl-xL. The interaction was very weak, and similar in both fractions. (Right) Bax-S184D was co-expressed with wild-type Bcl-xL. Like for BaxWT, the interaction with Bcl-xL occurred mostly in the mitochondrial fraction.
(B) Yeast cells co-expressing the three variants of Bax with Bcl-xL were grown in a small volume of SD-Lactate medium (2 mL) at the same O.D.600nm (0.2). 1% galactose was added to induce the expression of both proteins. 2 hours later, each culture was shared in half, and 20 µM ABT-737 (or an equivalent volume of DMSO) was added. The O.D.600nm was measured after 24 hours and the ratios between the presence and the absence of ABT-737 were plotted. The ratios did not change significantly after 36 hours.