Figure 1. FIGURE 1: Screening of an E. histolytica protein extract with an antibody against human soluble hTNFR1.
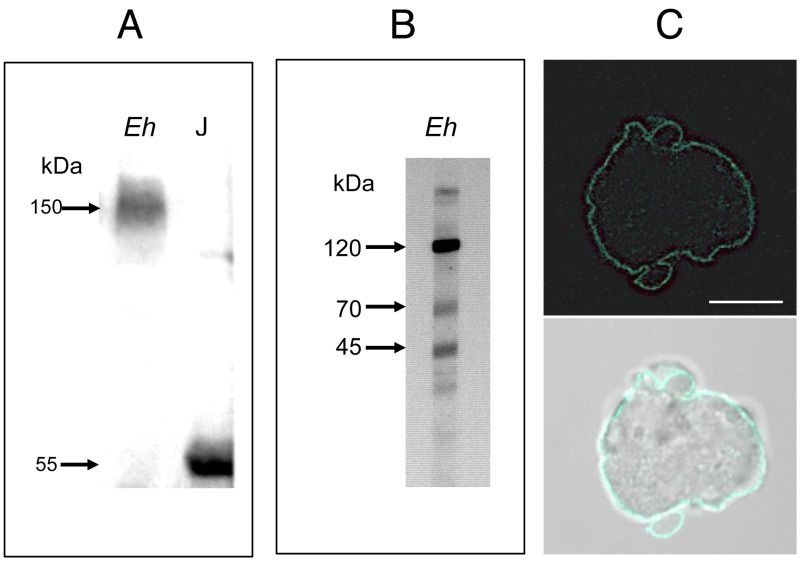
(A) Polyacrylamide gel electrophoresis under non-reducing conditions, and a Western blot analysis. Crude lysates of E. histolytica (Eh) and Jurkat (J) cells were loaded in the indicated lane (load: 20 µg of protein). Probing with the anti-hTNFR1 antibody revealed a single 150 kDa protein in E. histolytica and a single 55 kDa protein in Jurkat cells. (B) Polyacrylamide gel electrophoresis under reducing conditions, and a Western blot analysis. In a crude lysate from E. histolytica¸ three proteins (at approximately 120 kDa, 70 kDa and 45 kDa) were detected. (C) Entire trophozoites were fixed and stained with goat anti-hTNFR1 and anti-goat AlexaFluor488 in order to reveal the localization of amoebic proteins sharing an epitope with hTNFR1. Scale bar: 10 µm.
