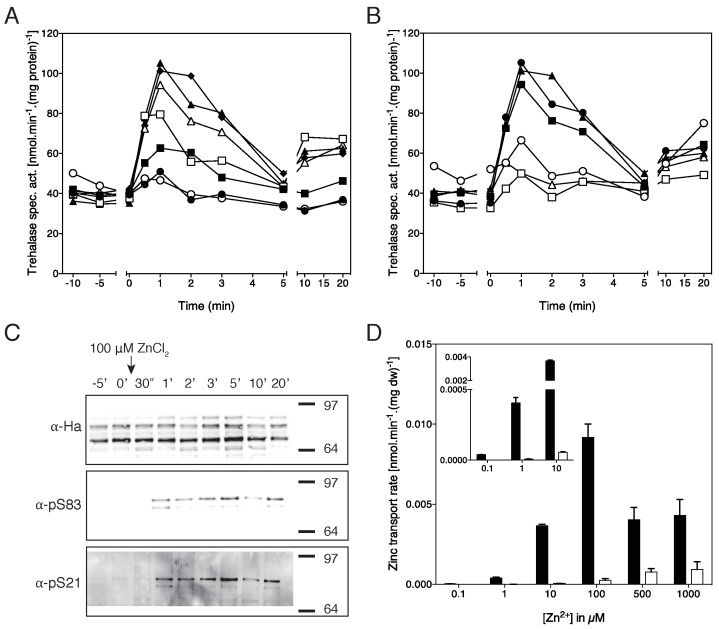
Figure 5. FIGURE 5: Re-addition of zinc to zinc-deprived cells triggers Zrt1-dependent activation of the PKA target trehalase.
(A) Activation of trehalase in the wild-type strain after addition of different concentrations of ZnCl2 to cells deprived for zinc for two days in the presence of the EDTA chelator. 100 nM (open circles), 500 nM (closed squares), 1 μM (open squares), 10 μM (closed triangles), 100 μM (open triangles), 1 mM (closed diamonds) ZnCl2 and starvation medium as negative control (closed circles).
(B) Activation of trehalase in wild-type (closed symbols) and zrt1Δ (open symbols) strains after addition of different concentrations of ZnCl2 to cells deprived for zinc for two days in the presence of the EDTA chelator. 1 μM (circles), 100 μM (squares) and 1 mM (triangles). (A,B) All experiments were performed 3-5 times, representative results are shown.
(C) Phosphorylation of trehalase on the amino acid residues S21 and S83 as detected with phosphospecific antibodies in response to addition of 100 μM ZnCl2 to cells of the wild type strain deprived for zinc for two days in the presence of the EDTA chelator. The trehalase-HA construct was expressed from the pYX212-NTH1-HA (LEU2) plasmid in the BY4742-strain.
(D) Uptake of different concentrations of Zn65Cl2 in the wild type (black bars) and zrt1Δ strains (white bars), n = 2. The inset shows the uptake at the lower concentrations in enlarged format.