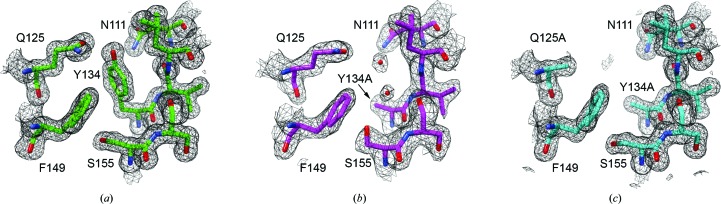
Figure 2.
2F o − F c electron-density maps contoured at 1σ near the mutation site. (a) shows the low-salt HpmA265 structure in green. (b) shows the low-salt Y134A structure in magenta and clear density for two ordered water molecules in place of the tyrosine side chain. (c) shows the low-salt AA double-mutant structure in cyan. Despite the loss of these two side chains the overall structure remains unaffected, and no electron density is observed that may account for ordered water molecules. Fig. 2 was created with the UCSF Chimera molecular visualization program (Pettersen et al., 2004 ▸).