Abstract
Discovery of sensitizing mutations in epidermal growth factor receptor (EGFR) and the subsequent development of EGFR tyrosine kinase inhibitors (TKIs) have substantially changed the treatment of lung cancer. First-line treatment with EGFR TKIs (gefitinib, erlotinib and afatinib) has demonstrated a superior response rate and progression-free survival (PFS) compared with chemotherapy in EGFR-mutation positive patients. However, a number of open questions remain, such as choice between the three EGFR TKIs licensed, treatment of patients unsuitable for chemotherapy due to morbidity or advanced age, management of acquired resistance and optimal biological sample to determine EGFR status. Recently the first head-to-head trial comparing gefitinib and afatinib (LUX-Lung 7) has been reported. Moreover, third-generation EGFR TKIs such as osimertinib, rociletinib, olmutinib and ASP8273, with preferential activity against T790M mutant tumours, the commonest resistance mechanism to EGFR TKIs, have shown promising results in early clinical trials, with osimertinib now licensed. In this review, we summarize latest advances in the treatment of EGFR-mutation positive patients focusing on controversial areas and emerging challenges to optimally treat these patients in the future.
Keywords: EGFR, mutation, non-small cell lung carcinoma, tyrosine kinase inhibitors
Introduction
In the last decade, the identification of epidermal growth factor receptor (EGFR) mutations and the development of molecular targeted therapies have launched the era of precision medicine in non-small cell lung cancer (NSCLC). EGFR mutations have been described in up to 17% of White patients with nonsquamous NSCLC, mostly adenocarcinomas and never-smokers [Rosell et al. 2009; Kris et al. 2014], and is three times more common in Asians for reasons still unknown. These somatic mutations mainly target exons 18–21 of EGFR, which encodes part of the tyrosine kinase (TK) domain of the gene and are clustered around the adenosine triphosphate (ATP)-binding pocket. The most common EGFR mutations are exon 19 deletions (del19) and exon 21 L858R substitutions (45–82% and 30%, respectively), that are commonly referred to as ‘sensitizing mutations’ as they confer sensitivity to TK inhibitors (TKIs), and constitute approximately 80–90% of EGFR mutations in adenocarcinomas [Lynch et al. 2004]. Sensitizing mutations in exon 18 (G719C, G719S, G719A and S720F) and others in exon 21 (L861Q and L861R) are less common. Other mutations include exon 20 insertions and point mutations, which are associated to primary TKI resistance. Identification of these EGFR-activating mutations in NSCLC is the single most important predictor of response and outcome to EGFR TKIs. However, despite 10 years of using TKIs, a number of open questions remain about the management of such patients. Here, we review areas of controversy with the latest evidence.
Is overall survival a useful endpoint in first-line EGFR TKI trials?
Nowadays it is well established that TKIs are a standard first-line treatment for patients with NSCLC harbouring EGFR mutations; the first-generation TKIs gefitinib and erlotinib, and the second-generation TKI afatinib have both been licensed for this indication since 2009. In phase III trials of first-line chemotherapy or EGFR TKI in NSCLC patients with EGFR mutations, gefitinib and erlotinib show significant improvements in overall response rate (ORR) and progression-free survival (PFS), but not in overall survival (OS) [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012] (Table 1). All of these trials except EURTAC [Rosell et al. 2012] exclusively enrolled Asian patients. EURTAC, an open label study comparing platinum-gemcitabine/docetaxel doublet chemotherapy to erlotinib, showed an increase in ORR from 15–58% and a median PFS from 5.2–9.7 months [Rosell et al. 2012]. However, as with other gefitinib and erlotinib trials, no significant difference in OS was observed between treatment arms.
Table 1.
Phase III trials comparing EGFR TKIs with chemotherapy in first-line EGFR-mutation positive NSCLC patients.
| Author | Study | Agent | N EGFR + (PS2) |
ORR | Median PFS (months) |
PFS HR | OS (months) | OS HR | Crossover % |
|---|---|---|---|---|---|---|---|---|---|
| Mok et al. 2009 | IPASS | Gefitinib | 261 (10%) |
71.2% versus 47.3% | 9.8 versus 6.4 | 0.48 (0.36–0.64) |
21.6 versus 21.9 | 1.00 (0.76–1.33) |
39.5 |
| Han et al. 2012 | First-SIGNAL | Gefitinib | 42 (9%) |
84.6% versus 37.5% | 8.0 versus 6.3 | 0.54 (0.27–1.1) |
27.2 versus 25.6 | 1.04 (0.50–2.18) |
75 |
| Mitsudomi et al. 2010 | WJTOG 3405 | Gefitinib | 172 (0%) |
62.1% versus 32.2% | 9.2 versus 6.3 | 0.49 (0.34–0.71) |
30.9 versus NR | 1.25 (0.88–1.78) |
59.3 |
| Maemondo et al. 2010 | NEJGSG002 | Gefitinib | 230 (1%) |
73.7% versus 30.7% | 10.8 versus 5.4 | 0.30 (0.22–0.41) |
30.5 versus 23.6 | 0.89 (0.63–1.24) |
94.6 |
| Zhou et al. 2011 | OPTIMAL | Erlotinib | 154 (6%) |
83% versus 36% | 13.7 versus 4.6 | 0.16 (0.10–0.26) |
22.7 versus 28.9 | 1.04 (0.69–1.58) |
NA |
| Rosell et al. 2012 | EURTAC | Erlotinib | 174 (14%) |
58% versus 15% | 9.7 versus 5.2 | 0.47 (0.28–0.78) |
19.3 versus 19.5 | 0.93 (0.64–1.35) |
76 |
| Sequist et al. 2013 | LUX-Lung 3 | Afatinib | 345 (0%) |
56% versus 23% | 13.6 versus 6.9 | 0.47 (0.34–0.65) |
31.6 versus 28.2 | 0.78 (0.58–1.06) |
75 |
| Wu et al. 2014 | LUX-Lung 6 | Afatinib | 364 (0%) |
67% versus 23% | 11.0 versus 5.6 | 0.28 (0.20–0.39) |
23.6 versus 23.5 | 0.83 (0.62–1.09) |
56 |
EGFR, epidermal growth factor receptor; HR, hazard ratio; NA, not applicable; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase.
More recently, afatinib showed a clear benefit for ORR and PFS compared with cisplatin/pemetrexed (LUX-Lung 3) or cisplatin/gemcitabine (LUX-Lung 6) [Sequist et al. 2013; Wu et al. 2014] in EGFR-mutant NSCLC. Although these results are similar to those of first-generation TKIs, these trials are distinguished by several factors: LUX-lung trials are the largest phase III trials to date of first-line EGFR TKI with chemotherapy; PFS and ORR were assessed by independent radiology review; both common sensitizing mutations (del19/L858R) and uncommon mutation patients were enrolled; and LUX-Lung 3 recruited both Asian and non-Asian patients using a modern chemotherapy comparator (cisplatin/pemetrexed). Although OS between afatinib and chemotherapy was not statistically significant different in each trial, in a prespecified combined analysis of individual patient data from both trials for common mutation, afatinib demonstrated a statistically significant OS improvement compared with chemotherapy [27.3 versus 23.5 months, hazard ratio (HR) = 0.81, 95% confidence interval (CI): 0.66–0.99, p = 0.037] [Yang et al. 2015a].
Whilst OS has been traditionally considered the strongest endpoint for clinical research in oncology, one of the commonest reasons for failure to observe a survival gain after a PFS improvement is the influence of post-progression therapy [Booth and Eisenhauer, 2012]. In trials where both the activity of the investigational drug and crossover on progression is high, PFS may be a better endpoint. In the phase III trials comparing EGFR TKI with chemotherapy, crossover is a huge confounder for OS due to 57–98% of patients assigned to the chemotherapy arm receiving second- or third-line therapy with EGFR TKIs (Table 1).
Is there evidence of different efficacy between EGFR TKIs for first-line therapy?
Regarding direct comparisons, a head-to-head comparison of gefitinib and erlotinib has not been carried in the first-line setting of EGFR-mutated NSCLC patients. There have been two studies conducted in Asian patients which directly compared erlotinib and gefitinib, but were based on previously treated patients [Yang et al. 2015c; Urata et al. 2016]. Neither of these studies showed differences in PFS and OS between TKIs. Additionally, a retrospective matched-pair case-control study compared 121 patients treated with gefitinib with 121 patients treated with erlotinib [Lim et al. 2014]. Of the 242 patients, 63 (26%) received EGFR TKI as first-line therapy. There were no statistically significant differences with regard to PFS (median, 11.7 versus 9.6 months, p = 0.056) or ORR (76.9% versus 74.4% p = 0.575) in the whole population or for patients receiving erlotinib or gefitinib as first-line treatment (median PFS, 11.7 versus 14.5 months, p = 0.507; and ORR, 76.7% versus 90.0%, p = 0.431). Both gefitinib and erlotinib are anilinoquinazolines with a similar molecular structure and similar mechanism of action in binding to the EGFR ATP pocket, so it seems biologically unlikely that large differences in efficacy may be observed. Therefore, a specific trial to directly compare gefitinib and erlotinib in the first-line treatment for EGFR-mutant patients may be clinically unjustified.
Recently, the first direct comparison between EGFR TKIs in the first-line setting has been reported (LUX-Lung 7) [Park et al. 2016]. In this exploratory phase IIb trial, 319 patients with NSCLC and common mutations (del19/L858R) were randomized to receive afatinib or gefitinib. Three co-primary endpoints were selected: PFS by independent central review, time-to-treatment failure (TTF) and OS. Unlike a classical superiority-testing trial, no formal hypotheses were defined and the sample size was based on controlling the half-width of the 95% CI for the logged HR to 0.25 in both directions of PFS. Afatinib significantly prolonged median PFS (11.0 versus 10.9 months, HR = 0.73, 95% CI: 0.57–0.95, p = 0.017), median TTF (13.7 versus 11.5 months, HR = 0.73, 95% CI: 0.58–0.92, p = 0.0073) and ORR (70% versus 56%, p = 0.0083) compared with gefitinib. For the third co-primary endpoint, OS, data were immature but median OS was not statistically different between treatment arms (27.9 and 25 months). Interestingly, although median PFS was almost identical in both arms, after this point a separation of the curves were observed and exploratory Kaplan–Meier estimates of PFS were consistently markedly higher for afatinib compared with gefitinib at the 18 month (27.3% versus 15.2%) and 24 month (17.6% versus 7.6%) landmarks.
The superiority of afatinib versus gefitinib may be explained by the different mechanism of action between the first-generation TKI gefitinib, which reversibly binds to, and inhibits EGFR signalling, and the second-generation irreversible TKI afatinib, which irreversibly binds to and blocks signalling from all relevant HER family receptor homo- and heterodimers (including EGFR, HER2, HER3 and HER4) [Li et al. 2008; Solca et al. 2012]. Amongst the mechanisms of acquired resistance to TKIs, HER2 amplification and mutations have been observed in 10% and 2% of patients treated with erlotinib or gefitinib, respectively [Mazieres et al. 2013]. However, due to its pan-HER2 inhibitor activity, this mechanism has not been reported with afatinib to date. In a small study with 42 patients with acquired resistance to afatinib, neither small-cell or squamous-cell lung cancer transformation was observed, nor were other somatic mutations in PIK3CA, BRAF, HER2, KRAS, NRAS, MEK1, AKT2, LKB1 and JAK2 identified after treatment with first-generation TKIs [Wu et al. 2016]. However, T790M mutation, the major mechanism of acquired resistance to first-generation TKIs, is also detected in half of the patients with acquired afatinib resistance, being not dissimilar to that proportion observed with first-generation EGFR TKI-treated patients [Wu et al. 2016]. Albeit with limitations of scarce data, these potentially different mechanisms of acquired resistance during afatinib treatment may explain the separation between PFS curves with time in the LUX-Lung 7 trial. To date, a head-to-head comparison of afatinib versus erlotinib in first-line EGFR-mutated NSCLC patients has not been performed.
Dacomitinib is another pan-HER inhibitor that irreversibly binds to HER1/EGFR, HER2 and HER4 TKs. In a pooled subset analysis of EGFR-mutated patients from two randomized trials comparing dacomitinib with erlotinib in previously treated molecularly unselected NSCLC (ARCHER 1009 [ClinicalTrials.gov identifier: NCT01360554] and A7471028 [ClinicalTrials.gov identifier: NCT00769067]), no statistically differences in median PFS (14.6 versus 9.6, p = 0.146) or OS were observed (26.6 versus 23.2 months, p = 0.265) for dacomitinib versus erlotinib, respectively [Ramalingam et al. 2016b]. Results from ARCHER 1050, a phase III trial comparing dacomitinib with gefitinib as first-line therapy in EGFR-mutated patients is awaited to replicate findings from LUX-Lung 7 that irreversible TKIs are potentially superior to first-generation TKIs.
Are adverse events different according to the type of TKI?
Besides efficacy, toxicity is the other cornerstone upon which the choice of TKI should be based. Trials results suggest that toxicity profiles of TKIs are different. In the direct comparison of afatinib and gefitinib (LUX-Lung 7), the number of patients discontinuing treatment due to adverse events (AEs) was similar in each group. However, the most frequent drug-related AEs leading to discontinuation differed between the two groups: diarrhoea (3%) was the commonest reason for afatinib, whilst increased alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels (3%) and interstitial lung disease (ILD) (3%) were the commonest for gefitinib. Moreover, grade ⩾3 AEs were markedly more frequent for afatinib (31%) than gefitinib (18%).
Although a direct comparison of erlotinib with afatinib has not been performed in first-line EGFR-mutated patients, data from toxicity comparisons may be implied from the head-to-head study for second-line patients with squamous sub-type NSCLC (LUX-Lung 8) [Soria et al. 2015]. Here, drug discontinuations because of AEs were similar in each group, and grade 3–4 drug-related AEs were 25% for afatinib versus 17% for erlotinib. Furthermore, the toxicity profile was again different: grade 3–4 diarrhoea (11%) and stomatitis (4%) were more frequent for afatinib and rash or acne (10%) more frequent for erlotinib.
In the randomized phase III trial of Urata and colleagues comparing gefitinib and erlotinib in previously treated adenocarcinoma, the number of patients who discontinued treatment due to toxicity was similar (33 and 32 patients, respectively) [Urata et al. 2016]. As reported in previous trials, grade 3–4 rash toxicities were more frequent for erlotinib (18.1%) than for gefitinib (2.2%), whilst ALT/AST elevation was more common for gefitinib (6.2/13% for gefitinib versus 2.2/3.3% for erlotinib).
Icotinib, a novel first-generation EGFR TKI currently only available in China, was compared with gefitinib in NSCLC patients after one or two failed chemotherapy regimens [Shi et al. 2013]. The trial revealed that icotinib had equivalent efficacy and better tolerability than gefitinib: drug-related AEs were 61% and 70% for patients receiving icotinib and gefitinib, respectively (p = 0.04).
Recently, the results of a pooled analysis of the occurrence of severe AEs according to the type of EGFR TKI based on data extracted from 21 phase II and III trials published between 2006 and 2014 and including more than 1400 patients with EGFR-mutated NSCLC has been reported [Takeda et al. 2015]. The treatment discontinuation rate due to AEs was significantly more frequent for afatinib than for erlotinib (7.2% versus 4.1%, p = 0.04), as well as for gefitinib than for erlotinib (7.6% versus 4.1%, p = 0.032). However, these data may be biased by the inability to dose attenuate gefitinib. The commonest AEs for discontinuation for each TKI were: skin toxicity and diarrhoea for afatinib, hepatotoxicity for gefitinib and ILD for erlotinib. Grade ⩾3 rash and diarrhoea were significantly more frequent with afatinib than with erlotinib or gefitinib. The frequency of grade ⩾3 ILD was low and similar between all three EGFR TKIs (0.6–2.2%). Gefitinib was associated with significantly higher grade ⩾3 hepatotoxicity compared with erlotinib or afatinib.
In conclusion, physicians should fully consider the efficacy–toxicity balance for individual patients to select the appropriate TKI. Gefitinib, erlotinib and afatinib have different toxicity profiles and possibly differences in efficacy. Afatinib might be superior to first-generation TKIs but with slightly more rash and diarrhoea.
Which is the TKI of choice in unfit and elderly patients?
Phase III trials that led to the approval of gefitinib, erlotinib and afatinib as first-line treatment were conducted in patients suitable for platinum-doublet chemotherapy [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012; Sequist et al. 2013; Wu et al. 2014]. Patients medically unfit to receive standard first-line platinum-doublet chemotherapy owing to poor performance status (PS) or comorbidities were not represented in these trials. However, although data are scarce, unfit patients account for about 30% of newly diagnosed patients with advanced NSCLC [Davidoff et al. 2010]. So, special analysis for these populations underrepresented in these first-line trials is warranted.
Whilst LUX-Lung 3 and LUX-Lung 6 were restricted to patients with PS0–1 [Sequist et al. 2013; Wu et al. 2014], all of the gefitinib and erlotinib trials [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012] except one [Mitsudomi et al. 2010] also allowed enrolment of PS2 patients. However, the proportion of PS2 patients enrolled was small, only EURTAC included >10% PS2 patients [Rosell et al. 2012] (Table 1). Hence, the evidence for using TKIs from these studies is limited.
There has only been one small prospective phase II study of gefitinib that recruited 30 patients ineligible for chemotherapy according to PS or advanced age with EGFR mutations [Inoue et al. 2009]. A total of 22 patients had PS ⩾ 3, 68% of these showed a rapid improvement in PS at 1 month. ORR, PFS and OS were 66%, 6.5 months and 18.8 months, respectively. These results are much better than that observed in a retrospective analysis of 74 PS3–4 patients with advanced NSCLC who were treated with first-line gefitinib without EGFR mutational analysis. ORR, median PFS and median OS were 27%, 32 days and 61 days, respectively. Never smoking and adenocarcinoma were independent predictors of better PFS [Lee et al. 2010]. Toxicity for gefitinib in both studies was comparable with that observed in patients with good PS. Despite the difference in the results between these studies, TKI treatment in patients with poor PS and high suspicion of EGFR mutation according to clinical characteristics may be clinically justified, especially if there are no other therapeutic options suitable, as a randomized blinded trial versus placebo in this clinical setting would likely be difficult to recruit to.
TOPICAL, a randomized phase III trial in molecularly unselected advanced NSCLC patients unsuitable for chemotherapy due to PS ⩾ 2, presence of comorbidities or both, specifically addressed the efficacy of erlotinib compared with placebo [Lee et al. 2012]. Median OS was similar in both groups (3.7 versus 3.6 months for erlotinib and placebo, respectively). Although, a statistically significant improvement in median PFS was identified for erlotinib (2.8 versus 2.6 months, p = 0.019), this was not clinically significant. Nonetheless, population characteristics, demonstrating 40% of patients with squamous histology and 37% of smokers made the presence of EGFR mutations unlikely.
To date, no data from prospective studies of afatinib in patients with comorbidities unsuitable for chemotherapy are available. Evidence on the safety and efficacy of afatinib in medically unfit patients who have either suspected or confirmed EGFR mutation will come from the UK TIMELY trial [ClinicalTrials.gov identifier: NCT01415011].
Elderly patients were also underrepresented in the phase III clinical trials of first-line TKI. Whilst, approximately two-thirds of lung cancer cases are diagnosed in people aged ⩾65 years, and almost half of cases are diagnosed in patients aged >70 years [Owonikoko et al. 2007], fewer than 30% of patients enrolled into these trials were ⩾65 years of age [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012; Sequist et al. 2013; Wu et al. 2014]. Aging may be associated with lower body mass index, decreased physiologic reserve, polymorbidity and concomitant medications that might affect either TKI clearance or their efficacy and safety. Studies specifically evaluating TKIs in EGFR-mutated patients aged >70 years are therefore needed to better quantify and evaluate the TKI toxicity/efficacy balance.
Several observational studies and trials support the safety and efficacy of erlotinib in elderly patients, albeit in molecular unselected populations [Kurishima et al. 2013; Yoshioka et al. 2014], mainly informing the toxicity profile. Erlotinib was compared with oral vinorelbine prospectively in a randomized phase II trial in 113 advanced NSCLC patients aged ⩾70 years [Chen et al. 2012]. The most frequent treatment-related toxicities for erlotinib were no different to that observed in the phase III trial in general population: rash (64.9%), diarrhoea (29.8%), and mouth ulceration (14.0%). Median PFS and OS for EGFR-mutated patients treated with erlotinib were 8.4 months and 22.7 months, respectively.
Whereas safety and efficacy of gefitinib in EGFR-mutated patients aged ⩾70 years have been also confirmed for by several reports [Maemondo et al. 2012; Morikawa et al. 2015], studies specifically evaluating afatinib in elderly patients are limited [Rossi et al. 2016]. However, pharmacokinetic characteristics of afatinib are different from the first-generation TKIs. Whilst, gefitinib and erlotinib undergo extensive hepatic metabolism predominantly by cytochrome P450-dependent enzymes, in contrast, afatinib undergoes minimal biotransformation and oxidative cytochrome-mediated metabolism is of negligible importance. Thus, gefitinib and erlotinib have an important potential for interaction with other agents metabolized by, or are inhibitors/inducers of cytochrome-related enzymes. In a retrospective analysis of 49 patients with advanced NSCLC and EGFR mutations, median PFS was significantly longer in elderly patients treated with both gefitinib and afatinib in comparison with younger patients (12.6 and 5.6 months, respectively; p = 0.008). Median PFS was statistically superior in elderly patients treated with gefitinib compared with those receiving afatinib, although the small number of patients precludes any conclusion. This potentially superior activity of TKIs in elderly patients compared with young patients might be explained by the higher number of medications related to concomitant comorbidities that cause an increased plasma level of TKIs [Rossi et al. 2016].
Should we choose a different TKI depending on the type of EGFR mutation?
It has been extensively proved that EGFR sensitizing mutations (del19/L858R) predict the benefit of EGFR TKIs. However, whether the efficacy of TKIs varies between del19 and L858R mutations is still controversial. Several studies reported that advanced NSCLC patients with EGFR del19 had a higher benefit following treatment with gefitinib and erlotinib than those with L858R [Jackman, 2006; Riely, 2006; Rosell et al. 2012; Sequist et al. 2013], whilst others did not demonstrate this difference [Sequist et al. 2008; Mitsudomi et al. 2010; Fukuoka et al. 2011]. For instance, subgroup analysis of EURTAC showed that median PFS for both erlotinib and chemotherapy were superior for del19 than for L858R (del19: 11 versus 4.6 months for erlotinib/chemotherapy, HR = 0.30, 95% CI: 0.18–0.50, p < 0.00; L858R: 8.4 versus 6 months for erlotinib/chemotherapy, HR = 0.55, 95% CI: 0.29–1.02, p = 0.0539), although this has not formally been tested [Rosell et al. 2012]. Similarly, both LUX-Lung 3 and LUX-Lung 6 trials demonstrated PFS differences with afatinib on the basis of EGFR mutation type; PFS was most improved in del19 patients compared with L858R [Sequist et al. 2013; Wu et al. 2014] (Figure 1).
Figure 1.
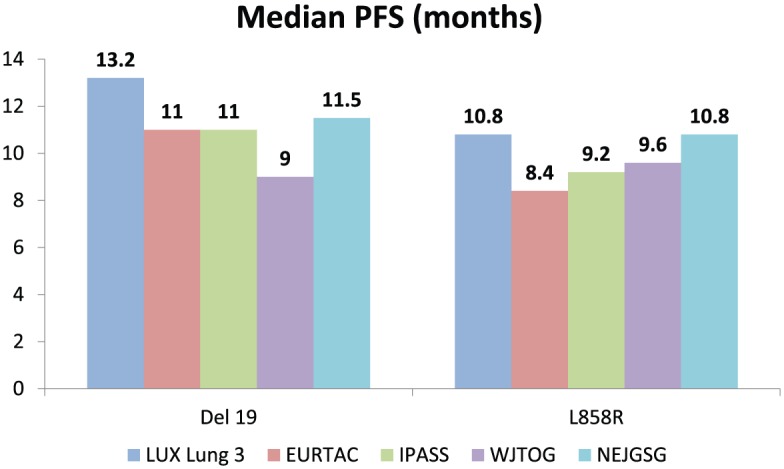
Median PFS and HR of EGFR TKIs versus chemotherapy according to the mutation type (del19 or L858R).
EGFR, epidermal growth factor receptor; HR, hazard ratio; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
There are three meta-analyses that have examined the impact of different EGFR mutations on PFS in the first-line setting [Wang et al. 2014; Zhang et al. 2014b; Lee et al. 2015a]. In all, patients with del19 had longer PFS with first-line EGFR TKIs compared with those with L858R. The results were similar through subgroup analyses stratified by the type of TKI [Zhang et al. 2014b]. However, afatinib showed the highest efficacy in patients harbouring del19 than those with L858R mutation (HR19/21 = 0.49, 95% CI: 0.21–1.17, p = 0.108), compared with gefitinib HR19/21 = 0.76, 95% CI: 0.47–1.21, p = 0.244) and erlotinib (HR19/21 = 0.53, 95% CI: 0.18–1.61, p = 0.264) [Zhang et al. 2014b] (Figure 1).
The LUX-Lung 3 and 6 trials are the only studies that demonstrated a differential OS difference between genotype. Here, an exploratory subgroup analyses by mutation genotype showed a statistically significant OS improvement at 12.2 and 13 months for afatinib in LUX-Lung 3 and LUX-Lung 6, respectively for del19, whereas no OS difference was observed in L858R patients [Yang et al. 2015a]. Pooled analysis of these trials was consistent the individual studies demonstrating an OS benefit in del19 patients only (HR = 0.59, 95% CI: 0.45–0.77, p = 0.0001; L858R HR = 1.25, 95% CI: 0.92–1.71, p = 0.16) [Yang et al. 2015a].
The mechanisms for these differences are currently unknown and several hypotheses might be considered: first, del19 might be more efficiently inhibited by EGFR TKIs due to an increased affinity for these than L858R mutations; second, T790M mutation (associated with acquired TKI resistance), might occur more frequently for L858R; and third, L858R could more frequently coexist with other uncommon EGFR mutations thereby affecting the EGFR kinase sensitivity to TKIs [Zhang et al. 2014b]. In summary, these results confirmed that del19 is a distinct disease form compared with L858R, implying that future studies should be stratified for mutation type.
Another related question is whether different EGFR TKIs should be used contingent on mutation type. In the head-to-head LUX-Lung 7 trial, afatinib resulted in a longer median PFS than gefitinib in both L858R (HR = 0.71, 95% CI: 0.47–1.06, p = 0.086) and del19 patients (HR = 0.76, 95% CI: 0.55–1.06, p = 0.107). Similarly, the ORR for L858R was 66% versus 42%, and in del19 73% versus 66% for afatinib versus gefitinib, respectively [Park et al. 2016]. Thus currently, tailoring treatment with afatinib or gefitinib on the basis of mutation subtype (del19 or L858R) is not indicated.
Data regarding sensitivity of tumours harbouring uncommon EGFR mutations such as E709X, G719X, L861Q, S768I and others to TKI are limited. Most phase III trials comparing first-line TKIs with chemotherapy were restricted to common mutations (del19/L858R). Only NEJ002 [Maemondo et al. 2010] and the LUX-Lung 3/6 [Sequist et al. 2013; Wu et al. 2014] included patients with uncommon mutations. In a post hoc analysis of NEJ002, gefitinib-treated patients with G719X or L861Q had a significantly shorter OS (11.9 versus 29.3 months; p < 0.001) than those with common mutations. However, the small number of uncommon mutations (4.4%) precludes establishing solid conclusions [Watanabe et al. 2014].
The largest dataset for uncommon mutations comes from post hoc analyses of pooled afatinib outcomes from LUX-Lung 3/6, where 11% of patients recruited had uncommon mutations, and LUX-Lung 2 (a single-arm phase II afatinib trial) [Yang et al. 2015b]. Here, patients were categorized as: point mutations or duplications in exon 18–21 (group 1); de novo T790M mutations alone or in combination with others (group 2); or exon 20 insertions (group 3). ORR was 71.1%, 14.3% and 8.3 % for group 1, 2 and 3, respectively. Median PFS and OS were 10.7 and 19.4 months for group 1, 2.9 and 14.9 months for group 2, and 2.7 and 9.2 months for group 3. For the most frequent uncommon mutations, the ORR for G719X was 77.8%, 56.3% for L861Q, and 100% with S768I.
Afatinib is therefore unique in showing activity in NSCLC patients with uncommon EGFR mutations, especially G719X, L861Q, and S768I from trial data.
Can we delay the development of acquired resistance to first-line TKIs?
Unfortunately, despite initial benefit, virtually all patients ultimately progress due to acquired resistance. Intra-tumour heterogeneity is particularly relevant for NSCLC, and it has been postulated as one of the main reasons for the incomplete disease response and acquired resistance observed, with the influence of drug therapy in the selection of cell clones demonstrated [Bai et al. 2012]. In a recent study, clones with EGFR mutations, wild-type EGFR clones and even cells with ALK translocations can be found in the same tumour [Cai et al. 2015]. The most common mechanism (60%) [Yu et al. 2013] of acquired resistance to first-generation EGFR TKIs is by clonal selection of the T790M allele. There are two theories that have been suggested regarding the appearance of the T790M mutation: ‘acquirement’ and ‘selection’ [Nguyen et al. 2009]. Initially it was described that patients with EGFR sensitizing mutations did not exhibit T790M mutation and only acquired this mutation after exposure to gefitinib or erlotinib [Pao et al. 2005]. However, in some cases, T790M mutation has been found in patients not treated with TKIs [Toyooka et al. 2005]. De novo T790M mutations detected by conventional methods are rare (1–8%) [Yu et al. 2013] and are more often associated with the L858R mutation and imply poorer prognosis [Toyooka et al. 2005], supporting the tumour heterogeneity theory of different cell populations and of TKI therapy positively selecting T790M clones. A recent study has also now confirmed both clonal selection and the de novo somatic acquisition of T790M as a new acquired resistance event [Hata et al. 2016].
Combination therapy with TKI and chemotherapy may therefore be an approach to attack heterogeneous cellular clones. However, a combined analysis of INTACT1 and INTACT2 trials (combination gefitinib–chemotherapy in molecularly unselected NSCLC patients) showed no OS improvement for additional gefitinib in a post hoc EGFR-mutant subset [Bell et al. 2005]. This lack of benefit could be explained by pharmacological antagonism when TKI and chemotherapy are administered concomitantly: gefitinib and erlotinib may arrest the cell cycle in the G1 phase and interfere with the cell cycle phase-related cytotoxic effects of chemotherapy. Hence, separating chemotherapy and erlotinib administration might avoid this [Davies et al. 2011]. This rationale was tested in the FASTACT 2 trial. In this phase III trial, 451 molecularly unselected NSCLC patients were randomized to chemotherapy [gemcitabine/platinum with intercalated erlotinib (d15–28) or placebo 4-weekly]. PFS was significantly prolonged for erlotinib combination therapy versus chemotherapy alone (PFS 7.6 versus 6 months, HR = 0.57, p < 0.0001). Analysis by EGFR mutation status identified that treatment benefit was noted only in EGFR-mutant patients both for PFS (16.8 versus 6.9 months, HR = 0.25, p < 0.0001) and OS (31.4 versus 20.6 months, HR = 0.48, p = 0.0092). Although small (n = 97), this is the only trial reporting a statistically significant OS gain for patients treated with combination erlotinib–chemotherapy versus chemotherapy alone [Wu et al. 2013]. However, the magnitude of the PFS and OS advantage contributed by intercalated erlotinib–chemotherapy compared with EGFR TKI monotherapy followed by chemotherapy alone remains unknown.
Bevacizumab, a recombinant humanized monoclonal antibody, binds to vascular endothelial growth factor A (VEGF-A), causing inhibition of tumour-induced angiogenesis. This different target might be synergistic with EGFR TKIs, especially through inhibiting EGFR TKI resistance [Naumov et al. 2009]. Following on the subset analysis of EGFR-mutant patients recruited to the BETA trial of erlotinib with/without bevacizumab demonstrating a higher median PFS with the combination of erlotinib and bevacizumab (17.1 months) compared with erlotinib alone (9.7 months) [Herbst et al. 2011], a Japanese randomized phase II study evaluated this combination. Here, an impressive 16 months median PFS for the combination versus 9.7 months for erlotinib monotherapy (HR = 0.54, p = 0.0015) was demonstrated [Seto et al. 2014]. Discontinuation of treatment because of AEs occurred at similar frequency in both groups. Grade 3–4 AEs were more frequent in the erlotinib plus bevacizumab group (91%) than the in the erlotinib group (53%). The most common grade ⩾3 AEs were rash (25% in the combination versus 19% monotherapy), hypertension (60% versus 10%), and proteinuria (8% versus none), with no new safety signals for bevacizumab observed.
A European single-arm phase II trial of bevacizumab-erlotinib (BELIEF; ETOP 2–11) in EGFR common mutations demonstrated a less impressive median PFS of 13.8 months, with no difference in outcome between del19 or L858R, but more prolonged PFS in those with T790M detected at baseline by an ultrasensitive method (PCR-PNA) than without (16 versus 10.5 months, respectively) [Stahel et al. 2015]. The European Medicines Agency (EMA) has recently approved this combination and a confirmatory randomized European trial is recruiting (BEVERLY [ClinicalTrials.gov identifier: NCT02633189]). Another recently published single-arm phase II study tested the combination of gefitinib plus bevacizumab in EGFR-mutant all-comers [Ichihara et al. 2015]. Median PFS was 14.4 months, with a significant difference between del19 and L858R genotypes (18.0 versus 9.4 months; p = 0.006).
In addition to bevacizumab, ramucirumab, another anti-angiogenic monoclonal antibody targeted against VEGF receptor 2, is being evaluated in a phase III trial in combination with erlotinib to evaluate its efficacy and safety in patients with common EGFR mutations (RELAY [ClinicalTrials.gov identifier: NCT02411448]).
Other mechanisms of resistance, such as HER2 and MET amplification, and PIK3CA mutations, were also reported [Sequist et al. 2011; Yu et al. 2013]. Targeting cMET receptor in combination with EGFR TKI is likely to predict better survival. However, trials combining MET inhibitors such as tivantinib or onartuzumab have been negative [Lin et al. 2014]. Nowadays, trials with new MET inhibitors are recruiting. Similarly, the rationale of combining EGFR and ERBB2 inhibitors is via various molecular interactions across their downstream signalling pathways. Afatinib, as irreversible ErbB family blocker may play a role in the delay of the development of resistance by this mechanism [De Grève et al. 2015].
What is the role of third-generation TKIs? Present and future
The most frequent molecular mechanism of acquired resistance observed is the development of the gatekeeper T790M point mutation (observed in up to 60% of cases) [Yu et al. 2013].
Although total EGFR blockade by the combination of afatinib and cetuximab showed a 29% ORR in a phase Ib trial for patients with acquired resistance to erlotinib, this combination had substantial toxicity with grade ⩾3 AEs observed in >20% of patients [Janjigian et al. 2014].
Third-generation mutation-specific EGFR TKIs, such as rociletinib, osimertinib (AZD9291), olmutinib (BI1482694/HM61713) and ASP8273, were specifically designed to inhibit EGFR in a covalent irreversible manner, with preferential activity against both T790M and classical sensitizing EGFR mutations, but with minimal activity against the EGFR wild-type allele. Rociletinib is no longer being developed after the United States Food and Drug Administration (FDA) considered the data from the phase I–II TIGER-X and TIGER-2 trials insufficient to recommend accelerated approval.
Osimertinib was tested in the phase I dose escalation trial (AURA [ClinicalTrials.gov identifier: NCT01802632]) at doses of 20–240 mg once daily in 31 patients with EGFR-mutated NSCLC with disease progression following treatment with first-generation EGFR TKIs not selected by T790M status and 222 additional patients were included in the expansion cohorts according to prospective T790M status (re-biopsy at enrolment was required). The maximal tolerated dose was not reached at any dose level and 80 mg daily was the recommended dose to maximize efficacy and minimize skin and gastrointestinal toxicity. In the entire 239 evaluable patients, an ORR of 51% was observed, with a median PFS of 8.4 months [Jänne et al. 2015]. In T790M-positive patients, a 61% ORR and 9.6 month PFS was observed. In contrast, in T790M negative patients, ORR was 21% and PFS was 2.8 months. As observed in first- and second-generation TKIs ORR was higher in del19/T790M-positive tumours than in L858R/T790M-positive tumours (64% versus 57%, respectively). No dose-limiting toxicities were reported at any dose level. The commonest AEs were mainly grade 1–2: diarrhoea (47% all grade), rash (40%), nausea (22%) and poor appetite (21%). Grade ⩾3 AEs were 32%. Therefore, data from this study suggest that T790M is not only prognostic but also a predictive biomarker, and hence osimertinib was further developed in this group only.
A recent report of data from the phase I dose expansion cohort (AURA P1 [ClinicalTrials.gov identifier: NCT01802632]) and a pre-planned pooled analysis of two phase II studies (AURA extension [ClinicalTrials.gov identifier: NCT01802632] and AURA2 [ClinicalTrials.gov identifier: NCT02094261]), investigating osimertinib at 80 mg, confirmed the previous efficacy findings. In the AURA pooled phase II, by independent central review, an ORR of 66% and a median PFS of 11.0 months were observed in 411 T790M-positive patients progressing following EGFR TKI therapy, leading to EMA and FDA approvals. Again, most toxicities were grade 1–2, with rash grouped terms [41% (⩾G3 1%)] and diarrhoea [38% (⩾G3 1%)] [Yang et al. 2016a].
In addition, osimertinib has greater central nervous system penetration than first-generation TKIs [Ballard et al. 2016]. BLOOM [ClinicalTrials.gov identifier: NCT02228369] is a phase I multicentre trial to assess the safety and preliminary activity of osimertinib in patients with NSCLC with EGFR mutations who failed standard treatment and developed brain and leptomeningeal disease. Here, 11 out of 21 patients (52%), with leptomeningeal disease and stable extracranial disease treated with osimertinib showed shrinkage of brain lesions, with 7 (33%) confirmed partial responses [Yang et al. 2016b].
Similarly, olmutinib is approved in South Korea based on the result of the phase I/II HM-EMSI-101 trial showing an ORR of 62% in similarly pretreated patients [Lee et al. 2015b], with the confirmatory ELUXA2 phase II trial completed and results awaited.
The impressive results obtained with osimertinib have led to its evaluation in the first-line setting in an expansion cohort of AURA trial, at 80 mg (n = 30) or 160 mg (n = 30). EGFR mutation subtypes included del19 (37%) and L858R (40%); five patients were T790M positive. With median follow up of 16.6 months the confirmed ORR was 77% and median PFS 19.3 months [Ramalingam et al. 2016a]. These results appear to be promising but preliminary. The ongoing confirmatory randomized phase III trial FLAURA [ClinicalTrials.gov identifier: NCT02296125] is assessing the efficacy of osimertinib in first-line therapy in advanced NSCLC with EGFR common mutations compared with gefitinib or erlotinib. Moreover, the efficacy of osimertinib in patients with EGFR mutations and the EGFR T790M mutation at diagnosis will be evaluated in the AZENT trial [ClinicalTrials.gov identifier: NCT02841579].
Head-to-head comparison studies between a third-generation and first-generation TKIs will likely help determine the role of T790M-specific TKIs in the first-line setting, potentially as a way to attack tumour heterogeneity. Currently, several questions remain open such as whether sequencing first/second-generation TKIs followed by osimertinib may be superior for OS compared with commencing with first-line osimertinib. Moreover, which patients may get greater benefit of osimetinib in the first-line setting; whether this is independent of baseline T790M status. Ultrasensitive methodologies for detecting and quantification T790M might potentially be useful to determine over what absolute level T790M mutant alleles benefit from osimertinib [Watanabe et al. 2015].
Is tissue necessary to treat patients with EGFR mutations or are liquid biopsies adequate?
The study of resistance mechanisms to optimize acquired resistance management has provided a clinical rationale for re-biopsy. This impetus has increased with the higher efficacy observed with third-generation EGFR TKIs in patients harbouring T790M. Although re-biopsy is technically feasible in 50–90% of patients [Chouaid et al. 2014; Bosc et al. 2015; Hasegawa et al. 2015; Kawamura et al. 2016], this remains a challenging task in some cases because of the localization of lesions and patient comorbidities. Moreover, some patients refuse the procedure and up to 25% of the re-biopsies are non-informative as the sample provides no, or too few cells for pathological or molecular diagnosis [Chouaid et al. 2014]. Furthermore, biopsy results may be affected by spatial heterogeneity of the tumour [Zhang et al. 2014a; Cai et al. 2015]. Analysis of circulating tumour DNA (ctDNA), colloquially termed ‘liquid biopsies’, may be a feasible and suitable alternative for the identification of molecular alterations such as EGFR mutations. The use of ctDNA for the detection of EGFR mutations has been investigated in multiple studies demonstrating high specificity but low sensitivity compared with tissue EGFR status [Qiu et al. 2015].
Oxnard and colleagues performed analysis of plasma ctDNA of patients included in the AURA phase I trial using BEAMing technology. Both central tumour and plasma samples for diagnostic comparison were available in 216 patients. Sensitivity was 82–86% for sensitizing mutations and 70% for T790M mutation. ORR to treatment with osimertinib in patients with tumour and plasma T790M positive was 62% and 63%, respectively. However, ORR in T790M-negative tumours was 26%, whilst in patients with T790M-negative plasma was 46%, implying false-negative results. Similarly, tumour T790M positivity predicts benefit form osimertinib compared with tumour T790M-negative patients (PFS 9.7 versus 3.4 months, p < 0.001). T790M-positive plasma also predicts for a prolonged PFS of 9.7 months. However, median PFS in plasma T790M-negative patients was longer than expected (8.2 months) again due to T790M plasma false negatives. Therefore, plasma T790M-negative status cannot replace tumour biopsy [Oxnard et al. 2016].
In another analysis of plasma T790M from patients enrolled in the AURA phase II studies (AURA extension and AURA 2) by using the COBAS test. Plasma and tissue-based COBAS testing were similarly sensitive and specific compared with a next-generation sequencing (NGS) reference method. Sensitivity was 75–85% for sensitizing mutations and 61% for T790M mutation. ORR to osimertinib was similar in ctDNA T790M-positive patients and tissue positive patients (64% and 66%, respectively) [Jenkins et al. 2016]. Recently, Wakelee and colleagues reported EGFR genotyping analysis of matched urine, plasma and tissue form patients treated with rociletinib. Therascreen, BEAMing and Trovera quantitative NGS assays were used for tissue, plasma and urine analysis. Both plasma and urine sensitivity was 81%. From 181 samples matched for T790M result in plasma, urine and tissue, 104 (57%) were positive in all samples types. In T790M-positive patients, ORR and median PFS were similar independent of sample type (plasma, urine or tissue) from which T790M status was identified [Wakelee et al. 2016].
Indeed, plasma ctDNA analysis to determine T790M status is a minimally invasive effective method that may potentially avoid tumour biopsies. However, given the limited sensitivity, failure to identify T790M should be followed up with tissue verification. Moreover, given the molecular heterogeneity identified in tissue, ctDNA may potentially detect T790M missed on standard tumour biopsies [Tan et al. 2016]. Based in these results a new paradigm for use of plasma diagnostics can be proposed (Figure 2).
Figure 2.
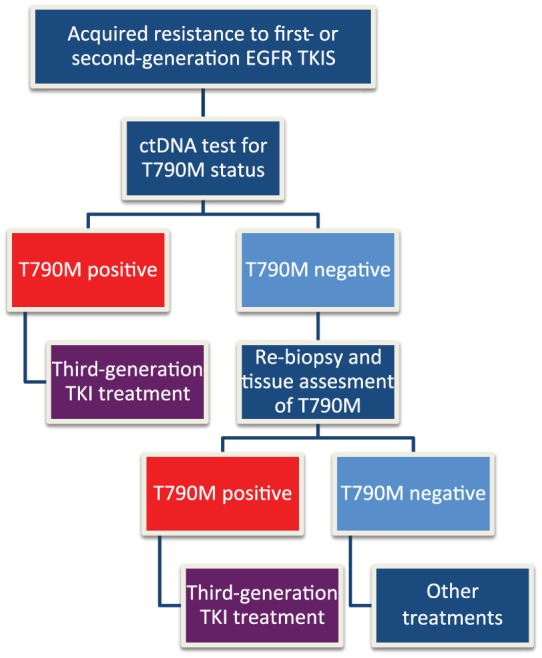
Algorithm to test T790M mutation integrating ctDNA in patients progressing to first- or second-generation EGFR TKIs.
ctDNA, circulating tumour DNA; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase.
Conclusion
Gefitinib, erlotinib and afatinib have dramatically changed the prognosis of EGFR-mutant NSCLC, showing significant improvements in ORR from 23–47% to 58–85% and PFS from 4.6–6.4 to 8–13.7 months compared with chemotherapy [Mok et al. 2009; Maemondo et al. 2010; Mitsudomi et al. 2010; Zhou et al. 2011; Han et al. 2012; Rosell et al. 2012; Sequist et al. 2013; Wu et al. 2014]. Despite these promising results, tumours invariably develop acquired resistance to EGFR TKIs. In addition, approximately 20% of patients with EGFR mutations exhibit de novo resistance and do not respond to EGFR TKI therapy. Although several mechanisms of acquired resistance have been reported, the most common type is T790M (observed in up to 60% of cases) [Yu et al. 2013].
Efforts have been made to answer what is the best EGFR TKIs to use first-line. LUX-Lung 7 has potentially shown superior efficacy for afatinib over gefitinib but at the cost of toxicity and results of the head-to-head dacomitinib versus gefitinib trial are awaited.
Moreover, result of the combination of erlotinib–bevacizumab is promising and may be a strategy to prolong PFS delaying the development of acquired resistance, especially in patients with T790M mutation. However, resistance mechanisms after progression to the combination are not known.
The emergence of third-generation TKIs has changed the whole treatment paradigm. Osimertinib has demonstrated an ORR of 66% and a median PFS of 11.0 months in patients who had progressed following EGFR TKI therapy through the T790M mutation. These impressive results have led to evaluating the efficacy of third-generation TKIs in first-line therapy (FLAURA study ongoing). Nevertheless, despite the positive results, acquired resistance to osimertinib emerges. The most frequent mechanism is the presence of a novel EGFR C797S gatekeeper mutation [Thress et al. 2015]. Moreover, it was recently reported that if C797S develops in T790M wild-type cells, the cells are resistant to third-generation TKIs, but retain sensitivity to first-generation TKIs [Niederst et al. 2015]. Therefore, it is unknown whether upfront osimertinib will be more effective than, in sequence, following first-generation TKI use and will delay the emergence of T790M mutations or, conversely, sequential TKI treatment will be superior to target different cell populations. Furthermore, patients who develop acquired resistance to osimertinib will need further treatments and disease aggressiveness in this setting is currently unknown.
The higher efficacy of the third-generation EGFR TKI in patients harbouring T790M has made re-biopsy necessary and has changed the management of lung cancer patients. Plasma ctDNA is a minimally invasive method for studying EGFR genotyping and could be a primary screen for T790M mutation. However, further efforts are needed to increase the sensitivity of these methodologies to reduce the risk of false-negative results.
Finally, the results of osimertinib in metastatic NSCLC have led to the assessment of osimertinib efficacy in the adjuvant setting. The ADAURA study [ClinicalTrials.gov identifier: NCT02511106], a phase III to assess the efficacy and safety of AZD9291 versus placebo, in patients with EGFR-mutation positive stage Ib–IIIa NSCLC, following complete tumour resection with or without adjuvant chemotherapy, is recruiting.
Ongoing clinical trials with third-generation EGFR TKIs will confirm their efficacy and will define the best approach in EGFR-mutation positive patients.
Acknowledgments
OJ acknowledges the Spanish Society of Medical Oncology (SEOM) and CRIS Cancer Foundation, Madrid (Spain), to support the collaboration with the Royal Marsden Hospital, UK. SP acknowledges UK National Health Service funding to the Royal Marsden Hospital / Institute of Cancer Research NIHR-Biomedical Research Centre, UK.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: SP is a consultant to AstraZeneca and Boehringer Ingelheim and has received travel expenses from Boehringer Ingelheim. OJ has no conflicts of interest to declare.
Contributor Information
Oscar Juan, Department of Medical Oncology, University Hospital La Fe, Valencia, Spain.
Sanjay Popat, Royal Marsden Hospital, London SW3 6JJ, UK.
References
- Bai H., Wang Z., Chen K., Zhao J., Lee J., Wang S., et al. (2012) Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol 30: 3077–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard P., Yates J., Yang Z., Kim D., Yang J., Cantarini M., et al. (2016) Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 22: 5130–5140. [DOI] [PubMed] [Google Scholar]
- Bell D., Lynch T., Haserlat S., Harris P., Okimoto R., Brannigan B., et al. (2005) Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 23: 8081–8092. [DOI] [PubMed] [Google Scholar]
- Booth C., Eisenhauer E. (2012) Progression-free survival: meaningful or simply measurable? J Clin Oncol 30: 1030–1033. [DOI] [PubMed] [Google Scholar]
- Bosc C., Ferretti G., Cadranel J., Audigier-Valette C., Besse B., Barlesi F., et al. (2015) Rebiopsy during disease progression in patients treated by TKI for oncogene-addicted NSCLC. Target Oncol 10: 247–253. [DOI] [PubMed] [Google Scholar]
- Cai W., Lin D., Wu C., Li X., Zhao C., Zheng L., et al. (2015) Intratumoral heterogeneity of ALK-rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol 33: 3701–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tsai C., Fan W., Shih J., Liu S., Wu C., et al. (2012) Phase II randomized trial of erlotinib or vinorelbine in chemonaïve, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol 7: 412–418. [DOI] [PubMed] [Google Scholar]
- Chouaid C., Dujon C., Do P., Monnet I., Madroszyk A., Le Caër H., et al. (2014) Feasibility and clinical impact of re-biopsy in advanced non-small cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer 86: 170–173. [DOI] [PubMed] [Google Scholar]
- Davidoff A., Tang M., Seal B., Edelman M. (2010) Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 28: 2191–2197. [DOI] [PubMed] [Google Scholar]
- Davies A., Ho C., Lara P., Jr., Mack P., Gumerlock P., Gandara D. (2011) Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer 7: 385–388. [DOI] [PubMed] [Google Scholar]
- De Grève J., Moran T., Graas M., Galdermans D., Vuylsteke P., Canon J., et al. (2015) Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer 88: 63–69. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Wu Y., Thongprasert S., Sunpaweravong P., Leong S., Sriuranpong V., et al. (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29: 2866–2874. [DOI] [PubMed] [Google Scholar]
- Han J., Park K., Kim S., Lee D., Kim H., Kim H., et al. (2012) First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30: 1122–1128. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Sawa T., Futamura Y., Horiba A., Ishiguro T., Marui T., et al. (2015) Feasibility of rebiopsy in non-small cell lung cancer treated with epidermal growth factor receptor-tyrosine kinase inhibitors. Intern Med 54: 1977–1980. [DOI] [PubMed] [Google Scholar]
- Hata A., Niederst M., Archibald H., Gomez-Caraballo M., Siddiqui F., Mulvey H., et al. (2016) Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 22: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R., Ansari R., Bustin F., Flynn P., Hart L., Otterson G., et al. (2011) Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 377: 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara E., Hotta K., Nogami N., Kuyama S., Kishino D., Fujii M., et al. (2015) Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 10: 486–491. [DOI] [PubMed] [Google Scholar]
- Inoue A., Kobayashi K., Usui K., Maemondo M., Okinaga S., Mikami I., et al. (2009) First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27: 1394–1400. [DOI] [PubMed] [Google Scholar]
- Jackman D. (2006) Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 12: 3908–3914. [DOI] [PubMed] [Google Scholar]
- Janjigian Y., Smit E., Groen H., Horn L., Gettinger S., Camidge D., et al. (2014) Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 4: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne P., Yang J., Kim D., Planchard D., Ohe Y., Ramalingam S., et al. (2015) AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372: 1689–1699. [DOI] [PubMed] [Google Scholar]
- Jenkins S., Yang J., Ramalingam S., Yu K., Patel S., Weston S., et al. (2016) Plasma ctDNA analysis for detection of EGFR T790M mutation in patients (pts) with EGFR mutation-positive advanced non-small cell lung cancer (aNSCLC). Europena Lung Cancer Conference (ELCC), Geneve, Switzerland 13–16 April, 2016. Abstr 134O. [Google Scholar]
- Kawamura T., Kenmotsu H., Taira T., Omori S., Nakashima K., Wakuda K., et al. (2016) Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Sci 107: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris M., Johnson B., Berry L., Kwiatkowski D., Iafrate A., Wistuba I., et al. (2014) using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311: 1998–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurishima K., Satoh H., Kaburagi T., Nishimura Y., Shinohara Y., Inagaki M., et al. (2013) Erlotinib for elderly patients with non-small-cell lung cancer: Subset analysis from a population-based observational study by the Ibaraki Thoracic Integrative (POSITIVE) Research Group. Mol Clin Oncol 1: 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Wu Y., Ding P., Lord S., Inoue A., Zhou C., et al. (2015a) Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 33: 1958–1965. [DOI] [PubMed] [Google Scholar]
- Lee J., Park K., Han J., Lee K., Kim J., Cho E., et al. (2015b) Clinical activity and safety of the EGFR mutant-specific inhibitor, BI 1482694 (HM61713), in patients with T790M-positive NSCLC. Ann Oncol 26: 125–147. [Google Scholar]
- Lee S., Khan I., Upadhyay S., Lewanski C., Falk S., Skailes G., et al. (2012) First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 13: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim H., Han J., Yun T., Lee G., Kim H., et al. (2010) First-line gefitinib treatment for patients with advanced non-small cell lung cancer with poor performance status. J Thorac Oncol 5: 361–368. [DOI] [PubMed] [Google Scholar]
- Li D., Ambrogio L., Shimamura T., Kubo S., Takahashi M., Chirieac L., et al. (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27: 4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Lee J., Sun J., Ahn J., Park K., Ahn M. (2014) Comparison of clinical outcomes following gefitinib and erlotinib treatment in non-small-cell lung cancer patients harboring an epidermal growth factor receptor mutation in either exon 19 or 21. J Thorac Oncol 9: 506–511. [DOI] [PubMed] [Google Scholar]
- Lin Y., Wang X., Jin H. (2014) EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res 4: 411–435. [PMC free article] [PubMed] [Google Scholar]
- Lynch T., Bell D., Sordella R., Gurubhagavatula S., Okimoto R., Brannigan B., et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139. [DOI] [PubMed] [Google Scholar]
- Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- Maemondo M., Minegishi Y., Inoue A., Kobayashi K., Harada M., Okinaga S., et al. (2012) First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol 7: 1417–1422. [DOI] [PubMed] [Google Scholar]
- Mazieres J., Peters S., Lepage B., Cortot A., Barlesi F., Beau-Faller M., et al. (2013) Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31: 1997–2003. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121–128. [DOI] [PubMed] [Google Scholar]
- Mok T., Wu Y., Thongprasert S., Yang C., Chu D., Saijo N., et al. (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Minegishi Y., Inoue A., Maemondo M., Kobayashi K., Sugawara S., et al. (2015) First-line gefitinib for elderly patients with advanced NSCLC harboring EGFR mutations. A combined analysis of North East Japan Study Group studies. Expert Opin Pharmacother 16: 465–472. [DOI] [PubMed] [Google Scholar]
- Naumov G., Nilsson M., Cascone T., Briggs A., Straume O., Akslen L., et al. (2009) Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 15: 3484–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K., Kobayashi S., Costa D. (2009) Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 10: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederst M., Hu H., Mulvey H., Lockerman E., Garcia A., Piotrowska Z., et al. (2015) The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 21: 3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owonikoko T., Ragin C., Belani C., Oton A., Gooding W., Taioli E., et al. (2007) Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 25: 5570–5577. [DOI] [PubMed] [Google Scholar]
- Oxnard G., Thress K., Alden R., Lawrance R., Paweletz C., Cantarini M., et al. (2016) Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 34: 3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W., Miller V., Politi K., Riely G., Somwar R., Zakowski M., et al. (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Tan E., O’Byrne K., Zhang L., Boyer M., Mok T., et al. (2016) Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 17: 577–589. [DOI] [PubMed] [Google Scholar]
- Qiu M., Wang J., Xu Y., Ding X., Li M., Jiang F., et al. (2015) Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 24: 206–212. [DOI] [PubMed] [Google Scholar]
- Ramalingam S., O’Byrne K., Boyer M., Mok T., Jänne P., Zhang H., et al. (2016b) Dacomitinib versus erlotinib in patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC): pooled subset analyses from two randomized trials. Ann Oncol 27: 423–429. [DOI] [PubMed] [Google Scholar]
- Ramalingam S., Yang J., Lee C., Kurata T., Kim D., John T., et al. (2016a) LBA1_PR: osimertinib as first-line treatment for EGFR mutation-positive advanced NSCLC: updated efficacy and safety results from two phase I expansion cohorts. J Thorac Oncol 11: S152. [Google Scholar]
- Riely G. (2006) Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 12: 839–844. [DOI] [PubMed] [Google Scholar]
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246. [DOI] [PubMed] [Google Scholar]
- Rosell R., Moran T., Queralt C., Porta R., Cardenal F., Camps C., et al. (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361: 958–967. [DOI] [PubMed] [Google Scholar]
- Rossi S., D’Argento E., Schinzari G., Dadduzio V., Di Noia V., Cassano A., et al. (2016) Are TKIs favourable for the elderly with non-small-cell lung cancer? Oncotarget 7: 46871–46877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist L., Martins R., Spigel D., Grunberg S., Spira A., Jänne P., et al. (2008) First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 26: 2442–2449. [DOI] [PubMed] [Google Scholar]
- Sequist L., Waltman B., Dias-Santagata D., Digumarthy S., Turke A., Fidias P., et al. (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3: 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist L., Yang J., Yamamoto N., O’Byrne K., Hirsh V., Mok T., et al. (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- Seto T., Kato T., Nishio M., Goto K., Atagi S., Hosomi Y., et al. (2014) Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 15: 1236–1244. [DOI] [PubMed] [Google Scholar]
- Shi Y., Zhang L., Liu X., Zhou C., Zhang L., Zhang S., et al. (2013) Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Solca F., Dahl G., Zoephel A., Bader G., Sanderson M., Klein C., et al. (2012) Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 343: 342–350. [DOI] [PubMed] [Google Scholar]
- Soria J., Felip E., Cobo M., Lu S., Syrigos K., Lee K., et al. (2015) Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 16: 897–907. [DOI] [PubMed] [Google Scholar]
- Stahel R., Dafni U., Gautschi O., Felip E., Curioni-Fontecedro A., Peters S., et al. (2015) A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutations with and without T790M mutation. Spanish Lung Cancer Group and the European Thoracic Oncology Platform BELIEF trial. European Cancer Congress, September 25–29, Vienna. Abstract 3BA. [Google Scholar]
- Takeda M., Okamoto I., Nakagawa K. (2015) Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 88: 74–79. [DOI] [PubMed] [Google Scholar]
- Tan C., Cho B., Soo R. (2016) Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer. Lung Cancer 93: 59–68. [DOI] [PubMed] [Google Scholar]
- Thress K., Paweletz C., Felip E., Cho B., Stetson D., Dougherty B., et al. (2015) Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 21: 560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka S., Kiura K., Mitsudomi T. (2005) EGFR mutation and response of lung cancer to gefitinib. N Engl J Med 352: 2136. [DOI] [PubMed] [Google Scholar]
- Urata Y., Katakami N., Morita S., Kaji R., Yoshioka H., Seto T., et al. (2016) Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol 1–13. [DOI] [PubMed] [Google Scholar]
- Wakelee H., Gadgeel S., Goldman J., Reckamp K., Karlovich C., Melnikova V., Soria J. (2016) Epidermal growth factor receptor (EGFR) genotyping of matched urine, plasma and tumor tissue from non-small cell lung cancer (NSCLC) patients (pts) treated with rociletinib. J Clin Oncol 34: abstract 9001. [Google Scholar]
- Wang H., Huang J., Yu X., Han S., Yan X., Sun S., et al. (2014) Different efficacy of EGFR tyrosine kinase inhibitors and prognosis in patients with subtypes of EGFR-mutated advanced non-small cell lung cancer: a meta-analysis. J Cancer Res Clin Oncol 140: 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Kawaguchi T., Isa S., Ando M., Tamiya A., Kubo A., et al. (2015) Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-Small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res 21: 3552–3560. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Minegishi Y., Yoshizawa H., Maemondo M., Inoue A., Sugawara S., et al. (2014) Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 9: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Liu Y., Tsai M., Chang Y., Yu C., Yang P., et al. (2016) The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 7: 12404–12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Lee J., Thongprasert S., Yu C., Zhang L., Ladrera G., et al. (2013) Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 14: 777–786. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou C., Hu C., Feng J., Lu S., Huang Y., et al. (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15: 213–222. [DOI] [PubMed] [Google Scholar]
- Yang J., Kim D., Kim S., Cho B., Lee J., Ye X., et al. (2016b) Osimertinib activity in patients with leptomeningeal disease from non-small cell lung cancer: updated results from the BLOOM study. J Clin Oncol 34: abstract 9002. [Google Scholar]
- Yang J., Ramalingam S., Jänne P., Cantarini M., Mitsudomi T. (2016a) LBA2_PR: Osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol 11: S152–S153. [Google Scholar]
- Yang J., Sequist L., Geater S., Tsai C., Mok T., Schuler M., et al. (2015b) Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 16: 830–838. [DOI] [PubMed] [Google Scholar]
- Yang J., Wu Y., Schuler M., Sebastian M., Popat S., Yamamoto N., et al. (2015a) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16: 141–151. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhou Q., Yan H. (2015c) A randomized controlled trial of erlotinib versus gefitinib in advanced non-small-cell lung cancer harboring EGFR exon 19 or 21 mutations (CTONG0901). 16th World Conference on Lung Cancer; Denver, CO, USA; Sept 6–9, 2015. Abstr 16.13. [Google Scholar]
- Yoshioka H., Komuta K., Imamura F., Kudoh S., Seki A., Fukuoka M. (2014) Efficacy and safety of erlotinib in elderly patients in the phase IV POLARSTAR surveillance study of Japanese patients with non-small-cell lung cancer. Lung Cancer 86: 201–206. [DOI] [PubMed] [Google Scholar]
- Yu H., Arcila M., Rekhtman N., Sima C., Zakowski M., Pao W., et al. (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Fujimoto J., Zhang J., Wedge D., Song X., Zhang J., et al. (2014a) Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 346: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sheng J., Kang S., Fang W., Yan Y., Hu Z., et al. (2014b) Patients with exon 19 deletion were associated with longer progression-free survival compared to those with l858r mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS ONE 9: e107161–e107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Wu Y., Chen G., Feng J., Liu X., Wang C., et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742. [DOI] [PubMed] [Google Scholar]


