Abstract
Given that drug abuse and dependence are common reasons for hospitalization, we aimed to derive and validate a model allowing early identification of life-threatening hospital admissions for drug dependence or abuse. Using the French National Hospital Discharge Data Base, we extracted 66,101 acute inpatient stays for substance abuse, dependence, mental disorders or poisoning associated with medicines or illicit drugs intake, recorded between January 1st, 2009 and December 31st, 2014. We split our study cohort at the center level to create a derivation cohort and a validation cohort. We developed a multivariate logistic model including patient’s age, sex, entrance mode and diagnosis as predictors of a composite primary outcome of in-hospital death or ICU admission. A total of 2,747 (4.2%) patients died or were admitted to ICU. The risk of death or ICU admission was mainly associated with the consumption of opioids, followed by cocaine and other narcotics. Particularly, methadone poisoning was associated with a substantial risk (OR: 35.70, 95% CI [26.94–47.32], P < 0.001). In the validation cohort, our model achieved good predictive properties in terms of calibration and discrimination (c-statistic: 0.847). This allows an accurate identification of life-threatening admissions in drug users to support an early and appropriate management.
Worldwide, illicit drug use and dependence are important contributors to the global burden of disease, accounting for 20 million disability-adjusted life years1. This burden is mainly associated with opioid dependence and increases in highest incomes countries1. In the United States, drug overdose deaths have increased significantly recent years2. A wide variety of healthcare, social and educational settings are involved in the identification and treatment of substance abuse- and dependence-related disorders3. Admissions to acute care hospitals for drug abuse or dependence are common and likely associated with complications.
Recently, it has been suggested that drug users were at higher risk for intensive care unit (ICU) admission than the general population4. Life-threatening hospital admissions need an early and appropriate management, and sometimes require supportive care5,6,7. In drug-overdosed patients, the immediate need for ICU monitoring can be assessed with tools such as the Glasgow coma score8. However, the latency of certain toxic syndromes can bias the initial evaluation and thus lead to overestimating the prognosis. Particularly, a secondary deterioration could be observed in patients initially admitted for behavioral disorders due to an acute intoxication, if no appropriate and early management is initiated. Accurately identifying potential life-threatening cases at admission is therefore of utmost importance and it could result in the respiratory and hemodynamic stabilization of the patient before ICU admission6. We propose to provide early prognostic information based on patient characteristics at hospital entrance, including the suspected responsible agent. To the best of our knowledge, such an approach has not been previously described in the medical literature for identifying life-threatening cases in drug users admitted to acute care hospitals.
In this study, we report both the derivation and the validation of a model allowing an early identification of life-threatening admissions for drug dependence or abuse (ILIADDA).
Methods
Ethics statement
This observational study was conducted according to the authorization given by the Agence pour le Traitement de l’Information Médicale (ATIH), which waived the need for patient’s consent according to the Enforcement (decree No. 94-666). All methods were carried out in accordance with relevant guidelines on prognosis study and the protocol was approved by the Commission Nationale de l’Informatique et des Libertés (agreement No. 1375062), as required by the French Protection Act. The data provided were de-identified.
Data source and study population
In France, the National Hospital Discharge Data Base (NHDDB) records complete anonymized information on all patients, in all French private and public hospitals. It provides discharge summaries of acute hospitalizations, which are grouped into Diagnosis-Related Groups (DRGs)9. For each DRG, an annual tariff is calculated at the national level based on average treatment costs.
We extracted all of the discharge summaries, recorded within the NHDDB from January 1st, 2009 to December 31st, 2014, reporting a DRG for substance abuse, dependence, mental disorders or poisoning associated with medicines or illicit drugs intake (see Supplemental Digital Content for the DRG codes). Admissions related to tobacco or alcohol disorders were not included.
Outcomes and predictors
We defined the primary endpoint as a composite outcome of ICU admission or in-hospital death.
In order to allow the clinician to predict the patient’s risk for the occurrence of the primary outcome at hospital admission, we considered the potential predictors as those available at entrance (time origin): age, sex, entrance by transfer and the diagnosis that motivated the hospitalization. This diagnosis at admission was recorded according to the International Classification of Diseases, 10th Revision (ICD-10)10.
Statistical analysis
For descriptive purposes, means (standard deviations, SD) and frequencies (percentages) were reported for continuous and categorical variables, respectively. In addition, we reported the temporal distribution of acute admissions related to drug dependence or abuse and we mapped the geographical distribution. The number of admissions by location was standardized on the number of inhabitants reported by the Institut National de la Statistique et des Études Économiques11. The map was created with © Géoclip 2015 – IGN GéoFla.
For predictive purposes, implementing a prognostic model in clinical practice routine must be preceded by three steps: the model derivation, the model validation and the clinical impact assessment12,13,14,15,16,17,18,19,20,21. In this study, we reported both the derivation and the validation of a model, according to the TRIPOD statement12,13. Given that the predictive performance of a model is likely to be optimistic relative to the data used for the model derivation, it must be evaluated on an independent set of patients. To this end, we split our study cohort into a derivation cohort and a validation cohort. There is a clear consensus as to the inefficiency of randomly splitting a cohort at the patient level, because it creates two very similar cohorts that only differ by chance12,13,22,23,24. Splitting by time (temporal or “narrow” validation) or by location (geographic or “broad” validation) is a more reliable approach12,13. To conduct a broad validation, we split the entire cohort at the center level (ratio 2:1 for derivation and validation, respectively). This allowed us to assess the performance of our model in centers, within which practices and measurements were likely to differ from those of the derivation cohort25.
In the derivation cohort, we performed a multivariate logistic regression (maximum likelihood estimation) adjusting the risk of death or ICU admission for several predictors. Due to the large sample size, selecting predictors according to the P-value would have led to a non-parsimonious model. Therefore, we limited the set of binary predictors to those for which 10 events (or more) occurred. For the continuous variable, we used a restricted cubic spline with three knots to handle the non-linearity of age effect. The efficiency of this procedure has been previously demonstrated for prediction modeling26. To provide a parsimonious and clinically usable final model, we removed the predictors associated with less than 0.25% of the multivariate model deviance, without damaging the predictive performance in terms of discrimination and calibration. Discrimination refers to the ability of the model to separate patients with different outcomes (i.e. those with life-threatening events versus those without), while calibration refers to the agreement between observed and predicted outcomes. In all analyses, multiple admissions for the same patient were considered as independent stays. Indeed, including the number of previous admissions per patient did not improve the model performance, while left-truncation dramatically reduced the sample size. In both the derivation cohort and the validation cohort, we reported the model discrimination (c-statistic: the area under the curve plotting sensitivity against 1 − specificity), overall accuracy (Brier score) and calibration (logistic regression of the observed outcome on the predictions for estimating the slope and intercept, and local weighted regression curve with span = 0.75 for graphical assessment). Confidence intervals of performance metrics were calculated by bootstrapping (500 iterations). All statistical analyses were carried out in R software (version 3.3).
Patient and funding organization involvement
No patients were involved in setting the research question; nor were they involved in the design of the study or the outcome measures. No funding organizations were involved in the study.
Results
Acute hospitalization in drug users
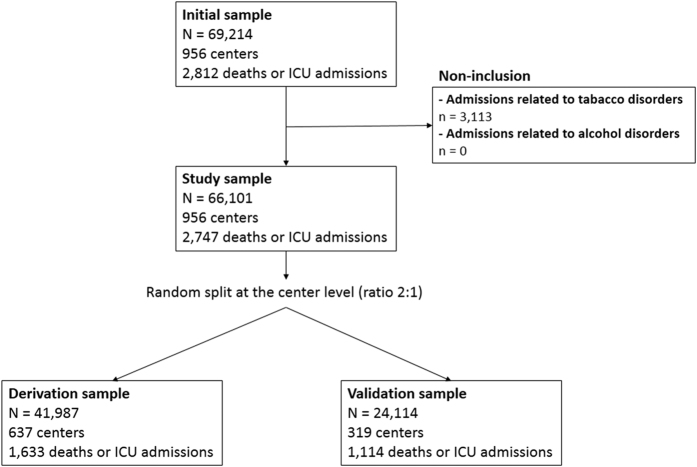
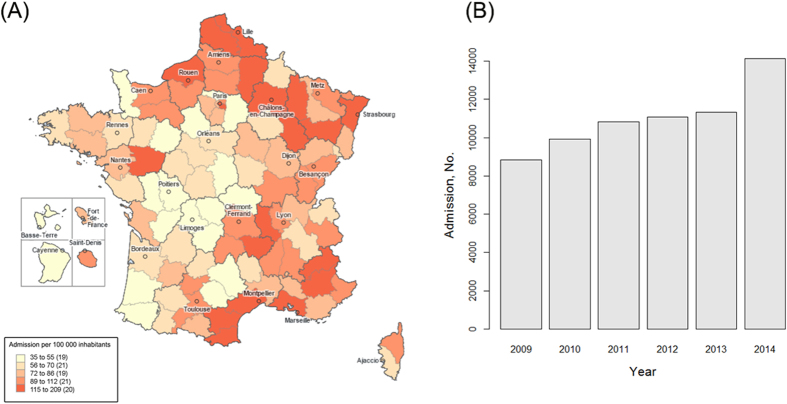
From January 2009 to December 2014, we reported a total of 66,101 acute admissions for drug abuse or dependence in 956 centers (Fig. 1). During this period, there was a substantial increase in the number of hospital admissions (from 8,834 admissions in 2009 to 14,118 admissions in 2014, +59.8%), which was associated with a widespread geographical distribution (Fig. 2). The mean age was 37.4 years (SD: 16.8) and 39,325 patients (59.5%) were male. Nine hundred and ninety-five patients (1.5%) entered by transfer from another structure. The mean length of stay was 3.6 days (SD: 5.7). A large majority of these admissions (61,875, 93.4%) occurred in public hospitals with a mean tariff of €1,704 (SD: 1,895).
Figure 1. Admissions flowchart.
Figure 2. Admission to acute care hospitals for drug abuse or dependence in France, according to the National Hospital Discharge Data Base (2009–2014).
The map was constructed with © Géoclip 2015 – IGN GéoFla (http://franceo3.geoclip.fr/#v=map6;l=fr;z=-1132292,6721213,2779367,1730915).
As summarized in Table 1, the main reason for admission was drug rehabilitation (19,319 admissions, 29.2%), including rehabilitation after drug abuse, drug detoxification, drug replacement therapy, drug withdrawal and treatment of withdrawal symptoms. Others were mostly related to the consumption of opioids (15,951 admissions, 24.1%), sedative or hypnotics (8,973 admissions, 13.6%), cannabinoids (7,445 admissions, 11.3%) and cocaine (2,709 admissions, 4.1%).
Table 1. Primary diagnoses at admission.
| Diagnosis (ICD-10 code) | No. (%) |
|---|---|
| Drug rehabilitation (Z503) | 19,319 (29.2%) |
| Opioids | 15,951 (24.1%) |
| Poisoning by opium (T400) | 214 (0.3%) |
| Poisoning by heroin (T401) | 548 (0.8%) |
| Poisoning by other opioids (T402) | 6,953 (10.5%) |
| Poisoning by methadone (T403) | 1,358 (2.1%) |
| Mental or behavioral disorders due to use of opioids (F11) | 6,871 (10.4%) |
| Finding of opiate drug in blood (R781) | 7 (0.0%) |
| Mental or behavioral disorders due to the use of sedatives or hypnotics (F13) | 8,973 (13.6%) |
| Cannabinoids | 7,445 (11.3%) |
| Poisoning by cannabis (derivatives) (T407) | 1,694 (2.6%) |
| Mental or behavioral disorders due to the use of cannabinoids (F12) | 5,751 (8.7%) |
| Cocaine | 2,709 (4.1%) |
| Poisoning by cocaine (T405) | 553 (0.8%) |
| Mental or behavioral disorders due to use of cocaine (F14) | 2,147 (3.2%) |
| Finding of cocaine in blood (R782) | 9 (0.0%) |
| Poisoning by other synthetic narcotics (T404) | 1,519 (2.3%) |
| Poisoning by other and unspecified narcotics (T406) | 1,072 (1.6%) |
| Poisoning by lysergide [LSD] (T408) | 110 (0.2%) |
| Poisoning by other and unspecified psychodysleptics (T409) | 434 (0.7%) |
| Mental or behavioral disorders due to the use of hallucinogens (F15) | 393 (0.6%) |
| Mental or behavioral disorders due to the use of other stimulants (F16) | 415 (0.6%) |
| Mental or behavioral disorders due to the use of volatile solvents (F18) | 176 (0.3%) |
| Mental or behavioral disorders due to multiple drug use and use of psychoactive substances (F19) | 5,785 (8.8%) |
| Others | 1,800 (2.7%) |
Risk of ICU admission or in-hospital death
In the entire cohort, the primary outcome occurred in 2,747 stays (4.2%), with 2,602 admissions to ICU and 211 in-hospital deaths. Of these deaths, 66 occurred in ICU (mortality rate in ICU: 2.5%). The mean SAPS II score at admission to ICU was equal to 35.0 (SD: 17.4).
For risk modeling, we split the study cohort at the hospital center level: 41,987 admissions in 637 centers were used for the model derivation, and 24,114 admissions to 319 centers for the model validation. A total of 1,633 (3.9%) and 1,114 (4.6%) deaths or ICU admissions occurred in the derivation cohort and in the validation cohort, respectively.
We reported a multivariate logistic model including 17 predictors (Table 2). Poisoning and acute intoxication by opioids were associated with a substantial increased risk of death or ICU admission: methadone poisoning OR: 35.70 (95% CI [26.94–47.32], P < 0.001), heroin poisoning OR: 22.72 (95% CI [15.84–32.61], P < 0.001), opium poisoning OR: 18.26 (95% CI [10.45–31.89], P < 0.001), poisoning by other opioids OR: 20.17 (95% CI [15.69–25.92], P < 0.001) and behavioral disorders due to an acute opioids intoxication OR: 17.61 (95% CI [13.49–22.97], P < 0.001). Poisoning and acute intoxication by narcotics other than opioids also increased the outcome risk: cocaine poisoning OR: 12.70 (95% CI [8.09–19.93], P < 0.001), behavioral disorders due to an acute cocaine intoxication OR: 3.12 (95% CI [1.69–5.79], P < 0.001), poisoning by other synthetic narcotics OR: 8.99 (95% CI [6.26–12.91], P < 0.001) and poisoning by other and unspecified narcotics OR: 18.97 (95% CI [13.26–27.14], P < 0.001). Other drugs had a lower, but still substantial effect on the risk of death or ICU admission (Table 2). In contrast, rehabilitation of drug users was associated with a decreased risk of occurrence of the primary outcome, OR: 0.18 (95% CI [0.11–0.29], P < 0.001), as well as the female sex, OR: 0.86 (95% CI [0.77–0.96], P < 0.001). The age effect followed an “n-shaped” curve with a maximal risk at 44 years old, OR: 7.98 (Supplemental Digital Content).
Table 2. Model for identifying life-threatening admissions for drug dependence or abuse (ILIADDA).
| Regression coefficient | Odds ratio [95% CI] | P-value | |
|---|---|---|---|
| Constant | −6.615 | ||
| Age (y) | 0.060 | 1.06 [1.05–1.07] | <0.001 |
| (Age)′ | −0.061 | 0.94 [0.93–0.95] | <0.001 |
| Female sex | −0.155 | 0.86 [0.77–0.96] | 0.006 |
| Transfer at entrance | 0.957 | 2.60 [1.99–3.41] | <0.001 |
| Reason for hospital admission (ICD-10 code) | |||
| Drug rehabilitation (Z503) | −1.712 | 0.18 [0.11–0.29] | <0.001 |
| Opioids | |||
| Poisoning by methadone (T403) | 3.575 | 35.70 [26.94–47.32] | <0.001 |
| Poisoning by heroin (T401) | 3.123 | 22.72 [15.84–32.61] | <0.001 |
| Poisoning by opium (T400) | 2.905 | 18.26 [10.45–31.89] | <0.001 |
| Poisoning by other opioids (T402) | 3.004 | 20.17 [15.69–25.92] | <0.001 |
| Mental and behavioral disorders due to use of opioids, acute intoxication (F110) | 2.868 | 17.61 [13.49–22.97] | <0.001 |
| Cocaine and other narcotics | |||
| Poisoning by cocaine (T405) | 2.542 | 12.70 [8.09–19.93] | <0.001 |
| Mental and behavioral disorders due to use of cocaine, acute intoxication (F140) | 1.139 | 3.12 [1.69–5.79] | <0.001 |
| Poisoning by other synthetic narcotics (T404) | 2.196 | 8.99 [6.26–12.91] | <0.001 |
| Poisoning by other and unspecified narcotics (T406) | 2.943 | 18.97 [13.26–27.14] | <0.001 |
| Others | |||
| Poisoning by cannabis (derivatives) (T407) | 1.207 | 3.34 [1.93–5.78] | <0.001 |
| Poisoning by other and unspecified psychodysleptics [hallucinogens] (T409) | 2.105 | 8.21 [4.71–14.30] | <0.001 |
| Mental and behavioral disorders due to use of sedatives or hypnotics, acute intoxication (F130) | 1.817 | 6.16 [4.73–8.02] | <0.001 |
| Mental and behavioral disorders due to multiple drug use and use of psychoactive substances, acute intoxication (F190) | 1.908 | 6.74 [4.89–9.28] | <0.001 |
A restricted cubic spline with three knots was used to describe the effects of age (knots at 19, 36 and 59 years).
The model can be used to predict the individual risk of a life-threatening event with the formula:
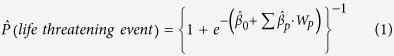 |
For each individual, Wp denotes his/her value for the pth predictor, with 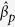 the corresponding estimated regression coefficient (i.e. logarithm of the OR of the pth predictor) and
the corresponding estimated regression coefficient (i.e. logarithm of the OR of the pth predictor) and 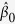 the estimated constant as reported in Table 2.
the estimated constant as reported in Table 2.
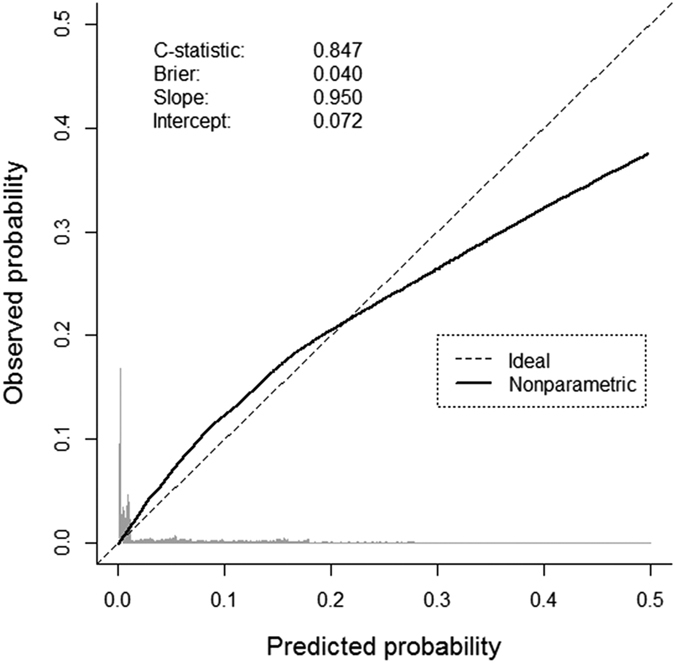
In the derivation cohort, our model achieved good predictive performances: c-statistic 0.851 (95% CI [0.844–0.859]) and Brier score 0.034 (95% CI [0.033–0.036]). This was confirmed in the validation cohort: c-statistic 0.847 (95% CI [0.838–0.855]) and Brier score 0.040 (95% CI [0.038–0.043]). The overall calibration slope in the validation cohort (0.950) depicted a slight over-fitting issue of the model development. Using a local weighted regression curve, we compared the predicted probabilities with the observed outcome frequencies (Fig. 3). In the validation cohort, we reported a small overestimation of the predicted probabilities beyond 0.2, which only concerned a few patients.
Figure 3. Calibration plot comparing predicted versus observed deaths or ICU admissions in the validation sample.
Discussion
We report the derivation and validation of a model, which demonstrates an accurate prediction of death or ICU admission in hospitalized drug users.
To the best of our knowledge, we provide the first model allowing an early identification of life-threatening cases in drug users admitted to acute care hospitals. This is of great importance as, in contrast with diagnosis scores (e.g., Glasgow coma score) that assess the immediate need for intensive care and are likely to vary according to the latency of the “toxidrome”, our model provides accurate prognostic information at hospital entrance on the risk of life-threatening events. In response to this clinical issue, our model includes only predictors that are readily available at hospital admission. Though we recognize that comorbidities, cognitive status, medications and biological markers are potential predictors, we did not take them into account in our model since these are not systematically investigated in every patient upon admittance. Had we considered those potential predictors would have led to limiting the usability of our model only to patients whose comorbidities, cognitive status, medications and biological markers are recorded at admission. Indeed, in the case of missing values regarding one of those predictors (i.e. absence of input while using the corresponding model formula) the predicted probability of life-threatening event would no longer be calculable. Without including those variables, our model still demonstrates good predictive properties in terms of discrimination and calibration. This might therefore be a useful tool for routine practice, supporting clinical decision-making to improve the early management of critically ill patients, who increasingly contribute to the global burden of healthcare.
Drug abuse or dependence is a common reason for admission into acute care hospitals. In our large French nationwide cohort, we found that 4.2% of these hospitalizations led to death or ICU admission. This is concordant with the 3% of empoisoned patients requiring critical care in the United States7. Although these life-threatening cases are limited, they require an early and appropriate management5,6,27, which motivated the development and validation of our model. Predictors include early diagnosis, which is commonly revealed by specific clinical syndromes (“toxidromes”) suggesting particular substances. Life-threatening cases typically correspond to male adults within the range of 30–50 years old and involving an acute intoxication or poisoning by opioids. A rapid identification is essential, as patient’s respiratory and hemodynamic stabilization can be achieved before ICU admission6. As previously reported in other Western countries28,29, methadone poisoning corresponds to the greatest risk, followed by the other opioids. Indeed, in France, the nationwide DRAMES study (Décès en Relation avec l’Abus de Médicaments et de Substances – Death Related to Medication and Substance Abuse) identified methadone as the main drug responsible for death, above heroin and cocaine30,31. This high risk associated to methadone poisoning might be related to its long duration of action. Hall and colleagues showed that drug overdose deaths were mostly associated with diversion of opioid analgesics, particularly methadone29. To address the problem of prescription drug abuse, the American College of Physicians has recently published a position paper that provides guidance to prescribers and policymakers32. According to recent American studies, there has been a downturn trend in opioid analgesics abuse within the decade, which was associated with an increased shift to heroin abuse in a more widespread geographical distribution33,34,35,36. In terms of prognosis, our data suggest that the risk of death or ICU admission associated with heroin poisoning is lower than with methadone poisoning, but higher than other opioids.
Following opioids, cocaine and other narcotics are the next most responsible for life-threatening admissions. The risk of death or ICU admission related to cocaine poisoning is about half of that associated with methadone, but is still substantial37. Other drugs responsible for life-threatening admissions are hallucinogens, sedatives or hypnotics, and cannabis or derivatives. In contrast to acute poisoning or intoxication, drug rehabilitation is associated with a reduced risk of outcome. Further studies are needed to demonstrate whether chronic care management is efficient38.
One of the strengths of our study is using a complete nationwide database. The large sample size enabled a broad model validation. It assessed the performances of our model on centers that were not used for the model derivation. All of these analyses were reported with transparency.
Our study has to be considered in the context of its limitations. First, the lack of sensitivity of the French medico-administrative database for identifying drug-dependent patients has been previously discussed39. However, this issue has been highlighted in the context of a routine detection of drug users. We recognize that the French NHDDB should not be used for drug user detection, as the population admitted to acute care hospitals does not reflect the overall drug-addicted population, who is likely to benefit from a wide variety of non-healthcare, social and educational settings. However, such a lack of sensitivity did not concern our study, since we focused on the most severe acute admissions, which were specifically motivated by substance abuse, dependence, mental disorders or poisoning associated with medicines or illicit drug intake. Use of this database should not give rise to an excessive number of false-positive cases, as the French NHDDB is primarily used for reimbursement purposes and is unlikely inclined to misdiagnoses39. This database records for each stay the diagnosis that resulted in hospitalization and the associated comorbidity (when applicable), which follow the International Classification of Diseases, 10th Revision. The granularity of this classification does not enable to describe the precise clinical spectrum of the disease and its evolution. Though medico-administrative databases can be regarded as relevant tools for describing admissions specifically related to illicit drugs consummation40, their potential use for clinical toxicology is still limited39,41,42. In certain situations, the information on drugs responsible for admission were limited to the drug classes stated by the ICD-10, which did not allow us to explore the risk related to some specific drugs, in particular, prescription opioids (e.g. oxycodone). Moreover, the history of drug abuse is not collected as such in the database, which is a limitation of our approach. Additionally, predictors such as individual socio-demographic characteristics were not available in our database. In spite of these limits, our model demonstrated good predictive performances. Nevertheless, the effect of its implementation on the decision-making process has not been measured, thus a further study is needed to assess its clinical impact, before implementation in routine clinical practice.
Conclusions
We report a simple model, which predicts the risk of death or ICU admission in drug users with good discriminative and calibration properties. This model, which includes patients’ characteristics at hospital admission might be useful in clinical practice for early identification of life-threatening cases.
Additional Information
How to cite this article: Nguyen, T.-L. et al. Identifying Life-Threatening Admissions for Drug Dependence or Abuse (ILIADDA): Derivation and Validation of a Model. Sci. Rep. 7, 44428; doi: 10.1038/srep44428 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We warmly thank Sarah Kabani for her valuable editing help.
Footnotes
The authors declare no competing financial interests.
Author Contributions Study concept and design: T.L.N., T.B., H.P., P.L. Analysis and interpretation: T.L.N., T.B., H.P., P.L. Drafting of the manuscript: T.L.N., T.B., H.P., P.L. Critical revision of the manuscript for important intellectual content: T.L.N., T.B., G.L.B., C.E., J.M.K., Y.L.M., H.P., P.L.
References
- Degenhardt L. et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet 382, 1564–1574, doi: 10.1016/S0140-6736(13)61530-5 (2013). [DOI] [PubMed] [Google Scholar]
- Rudd R. A., Aleshire N., Zibbell J. E. & Gladden R. M. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. Centers for Disease Control and Prevention — Morbidity and Mortality Weekly Report 64, 1378–1382 (2016). [DOI] [PubMed] [Google Scholar]
- McLellan A. T. & Meyers K. Contemporary addiction treatment: a review of systems problems for adults and adolescents. Biological psychiatry 56, 764–770, doi: 10.1016/j.biopsych.2004.06.018 (2004). [DOI] [PubMed] [Google Scholar]
- Suchyta M. R., Beck C. J., Key C. W., Jephson A. & Hopkins R. O. Substance dependence and psychiatric disorders are related to outcomes in a mixed ICU population. Intensive care medicine 34, 2264–2267, doi: 10.1007/s00134-008-1263-9 (2008). [DOI] [PubMed] [Google Scholar]
- Alapat P. M. & Zimmerman J. L. Toxicology in the critical care unit. Chest 133, 1006–1013, doi: 10.1378/chest.07-1840 (2008). [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L. Poisonings and overdoses in the intensive care unit: general and specific management issues. Critical care medicine 31, 2794–2801, doi: 10.1097/01.CCM.0000100123.50896.F0 (2003). [DOI] [PubMed] [Google Scholar]
- Mokhlesi B., Leiken J. B., Murray P. & Corbridge T. C. Adult toxicology in critical care: part I: general approach to the intoxicated patient. Chest 123, 577–592 (2003). [DOI] [PubMed] [Google Scholar]
- Hamad A. E., Al-Ghadban A., Carvounis C. P., Soliman E. & Coritsidis G. N. Predicting the need for medical intensive care monitoring in drug-overdosed patients. J Intensive Care Med 15, 321–328 (2000). [Google Scholar]
- Manuel des Groupes Homogènes de Malades. Ministère des Affaires Sociales et de la Santé. http://social-sante.gouv.fr/fichiers/bos/2014/sts_20140005_0003_p000.pdf (2014).
- World Health Organisation, International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (1992).
- Institut National de la Statistique et des Études Économiques. Recensement de la population. http://www.insee.fr/fr/bases-de-donnees/default.asp?page=recensement/resultats/2012/donnees-detaillees-recensement-2012.htm (2012).
- Collins G. S., Reitsma J. B., Altman D. G. & Moons K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Bmj 350, g7594, doi: 10.1136/bmj.g7594 (2015). [DOI] [PubMed] [Google Scholar]
- Moons K. G. et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Annals of internal medicine 162, W1–73, doi: 10.7326/M14-0698 (2015). [DOI] [PubMed] [Google Scholar]
- Hemingway H. et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. Bmj 346, e5595, doi: 10.1136/bmj.e5595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani A. D. et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. Bmj 346, e5793, doi: 10.1136/bmj.e5793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. D. et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS medicine 10, e1001380, doi: 10.1371/journal.pmed.1001380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E. W. et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS medicine 10, e1001381, doi: 10.1371/journal.pmed.1001381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D. G., Vergouwe Y., Royston P. & Moons K. G. Prognosis and prognostic research: validating a prognostic model. Bmj 338, b605, doi: 10.1136/bmj.b605 (2009). [DOI] [PubMed] [Google Scholar]
- Moons K. G., Altman D. G., Vergouwe Y. & Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. Bmj 338, b606, doi: 10.1136/bmj.b606 (2009). [DOI] [PubMed] [Google Scholar]
- Moons K. G., Royston P., Vergouwe Y., Grobbee D. E. & Altman D. G. Prognosis and prognostic research: what, why, and how? Bmj 338, b375, doi: 10.1136/bmj.b375 (2009). [DOI] [PubMed] [Google Scholar]
- Royston P., Moons K. G., Altman D. G. & Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. Bmj 338, b604, doi: 10.1136/bmj.b604 (2009). [DOI] [PubMed] [Google Scholar]
- Austin P. C. & Steyerberg E. W. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Statistical methods in medical research, doi: 10.1177/0962280214558972 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E. W., Bleeker S. E., Moll H. A., Grobbee D. E. & Moons K. G. Internal and external validation of predictive models: A simulation study of bias and precision in small samples. Journal of Clinical Epidemiology 56, 441–447, doi: 10.1016/s0895-4356(03)00047-7 (2003). [DOI] [PubMed] [Google Scholar]
- Steyerberg E. W. et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54, 774–781 (2001). [DOI] [PubMed] [Google Scholar]
- Le Manach Y. et al. Preoperative Score to Predict Postoperative Mortality (POSPOM): Derivation and Validation. Anesthesiology 124, 570–579, doi: 10.1097/ALN.0000000000000972 (2016). [DOI] [PubMed] [Google Scholar]
- Collins G. S., Ogundimu E. O., Cook J. A., Manach Y. L. & Altman D. G. Quantifying the impact of different approaches for handling continuous predictors on the performance of a prognostic model. Statistics in medicine 35, 4124–4135, doi: 10.1002/sim.6986 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhlesi B., Garimella P. S., Joffe A. & Velho V. Street drug abuse leading to critical illness. Intensive care medicine 30, 1526–1536, doi: 10.1007/s00134-004-2229-1 (2004). [DOI] [PubMed] [Google Scholar]
- O’Dowd A. Deaths from drug poisoning in English and Welsh men reach five year peak. Bmj 337, a1521, doi: 10.1136/bmj.a1521 (2008). [DOI] [PubMed] [Google Scholar]
- Hall A. J. et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. Jama 300, 2613–2620, doi: 10.1001/jama.2008.802 (2008). [DOI] [PubMed] [Google Scholar]
- Jouanjus E., Gibaja V., Kahn J. P., Haramburu F. & Daveluy A. Signal identification in addictovigilance: the functioning of the French system. Therapie 70, 123–131 (2015). [DOI] [PubMed] [Google Scholar]
- Richard N., Arditti J., Pépin G., Mallaret M. & Castot A. Drames (Décès en relation avec l′abus de médicaments et de substances): outil d’identification des décès liés à la drogue en France. BEH, 40–41 (2010). [Google Scholar]
- Kirschner N., Ginsburg J. & Sulmasy L. S. Health & Public Policy Committee of the American College of, P. Prescription drug abuse: executive summary of a policy position paper from the American College of Physicians. Annals of internal medicine 160, 198, doi: 10.7326/M13-2209 (2014). [DOI] [PubMed] [Google Scholar]
- Dart R. C. et al. Trends in opioid analgesic abuse and mortality in the United States. The New England journal of medicine 372, 241–248, doi: 10.1056/NEJMsa1406143 (2015). [DOI] [PubMed] [Google Scholar]
- Cicero T. J., Ellis M. S. & Harney J. Shifting Patterns of Prescription Opioid and Heroin Abuse in the United States. The New England journal of medicine 373, 1789–1790, doi: 10.1056/NEJMc1505541 (2015). [DOI] [PubMed] [Google Scholar]
- Cicero T. J., Ellis M. S., Surratt H. L. & Kurtz S. P. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry 71, 821–826, doi: 10.1001/jamapsychiatry.2014.366 (2014). [DOI] [PubMed] [Google Scholar]
- McCarthy M. Illicit drug use in the US holds steady, but heroin use is on rise. Bmj 347, f5544, doi: 10.1136/bmj.f5544 (2013). [DOI] [PubMed] [Google Scholar]
- Single E., Rehm J., Robson L. & Truong M. V. The relative risks and etiologic fractions of different causes of death and disease attributable to alcohol, tobacco and illicit drug use in Canada. CMAJ 162, 1669–1675 (2000). [PMC free article] [PubMed] [Google Scholar]
- Saitz R. et al. Chronic care management for dependence on alcohol and other drugs: the AHEAD randomized trial. Jama 310, 1156–1167, doi: 10.1001/jama.2013.277609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanjus E., Pourcel L., Saivin S., Molinier L. & Lapeyre-Mestre M. Use of multiple sources and capture-recapture method to estimate the frequency of hospitalizations related to drug abuse. Pharmacoepidemiology and drug safety 21, 733–741, doi: 10.1002/pds.3280 (2012). [DOI] [PubMed] [Google Scholar]
- Chisolm D. J. & Kelleher K. J. Admission to acute care hospitals for adolescent substance abuse: a national descriptive analysis. Substance abuse treatment, prevention, and policy 1, 17, doi: 10.1186/1747-597X-1-17 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daveluy A. et al. Identification of abuse and dependence cases through a hospital database. Pharmacoepidemiology and drug safety 21, 1344–1349, doi: 10.1002/pds.3369 (2012). [DOI] [PubMed] [Google Scholar]
- Jouanjus E., Leymarie F., Tubery M. & Lapeyre-Mestre M. Cannabis-related hospitalizations: unexpected serious events identified through hospital databases. British journal of clinical pharmacology 71, 758–765, doi: 10.1111/j.1365-2125.2010.03897.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.