Abstract
Background:
In cases of chronic thromboembolic pulmonary hypertension (CTEPH), referral for possible surgical intervention is important because surgery can be curative. Surgery necessitates cardiopulmonary bypass and deep circulatory arrest with pulmonary thrombectomy and bilateral endarterectomy (PTE). If surgery fails, lung transplant is the next best surgical option. Medical treatment is also an important adjunct.
Case Report:
A 35-year-old female presented 3 months after a pulmonary embolus was found to be completely occluding her left pulmonary artery. She was found to have pulmonary hypertension with a pulmonary artery pressure of 81/33 mmHg, with a mean pressure of 52 mmHg. The right atrial pressure was also severely elevated at 29 mmHg, and her echocardiogram revealed severe tricuspid regurgitation and severe right ventricular dysfunction. She underwent PTE and postoperatively was followed by the heart failure team. Her 6-minute walk distance improved from 396 meters at 1 month to 670 meters at 7 months, and her pulmonary artery pressure improved significantly to 55/17 mmHg with a mean pressure of 31 mmHg. The patient's right atrial pressure also improved significantly from 29 mmHg to 13 mmHg.
Conclusion:
CTEPH is likely underrecognized, and patients with pulmonary hypertension or a history of pulmonary embolism should be screened for CTEPH. This case illustrates the surgical treatment for CTEPH and discusses alternative and adjunctive treatments. Residual pulmonary hypertension after PTE occurs in approximately 35% of patients. Overall, 4-year mortality rates after surgery appear to be approximately 15%, and mortality rates correlate with the postoperative pulmonary vascular resistance. Recognition of chronic pulmonary thromboembolic disease as the etiology of pulmonary hypertension warrants evaluation for surgery.
Keywords: Endarterectomy, hypertension–pulmonary, lung transplantation, pulmonary embolism
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is a condition that complicates approximately 4% of cases of pulmonary embolism.1 CTEPH is defined as a precapillary condition associated with an occlusive thrombus or emboli after 3 months of therapeutic anticoagulation and a mean pulmonary artery pressure >25 mmHg.2 The incidence in the United States is estimated to be 500-2,500 cases annually.3 Surgical intervention should be considered first because although medical treatment has improved with new disease-modifying agents, patients treated medically have higher mortality rates and worse functional status than patients who undergo surgery.4 Consequently, the ideal treatment for CTEPH is surgery, with the first-line operation being the potentially curative pulmonary thrombectomy and bilateral endarterectomy (PTE). Bilateral lung transplant is a salvage therapy. The patient's anatomy and the location of the thrombus have a large impact on the decision to operate, with the key questions being accessibility and baseline pulmonary vascular resistance.5 However, accessibility is subjective, as experienced surgeons will operate on patients with subsegmental disease.6 Approximately two-thirds of patients with CTEPH have disease amenable to an operation, and 2-year survival rates are approximately 10% higher in patients who undergo an operation compared to those who do not.7 After successful PTE, the resolution of right heart dysfunction and pulmonary hypertension (PH) is often swift. Remodeling is usually complete by 3 months, and these results are most often permanent.8 PTE has the advantage of a lower mortality rate than lung transplant and does not preclude transplant if the patient experiences persistent PH that does not improve with medication.6
We present a case of surgical treatment for CTEPH and the patient's postoperative course.
CASE REPORT
A 35-year-old female was referred for a mass in her left pulmonary artery. Three months prior to referral, she had been evaluated at a community hospital for shortness of breath and was treated for pneumonia. Her clinical condition failed to improve, and she was evaluated further. Computed tomography scan showed a large left pleural effusion and an occlusive mass that was suspected to be a pulmonary embolus. Ultrasound was negative for deep vein thrombosis. The patient was started on therapeutic anticoagulation. She continued to have shortness of breath and exercise intolerance and was referred to us. Our preoperative workup included a pulmonary angiogram that demonstrated an occluded left pulmonary artery and evidence of layered thrombi in the right pulmonary arterial system. Right heart catheterization showed an elevated pulmonary artery pressure of 81/33 mmHg with a mean pressure of 52 mmHg, a right ventricle pressure of 90 mmHg, and a right atrial pressure that was severely elevated at 29 mmHg. Cardiac magnetic resonance imaging showed severe right ventricular dysfunction, severe tricuspid regurgitation, and an ejection fraction of 47%, similar to what was seen on the echocardiogram (Figure 1). The mass was biopsied with negative results for tumor. The patient had a negative hypercoagulable workup.
Figure 1.
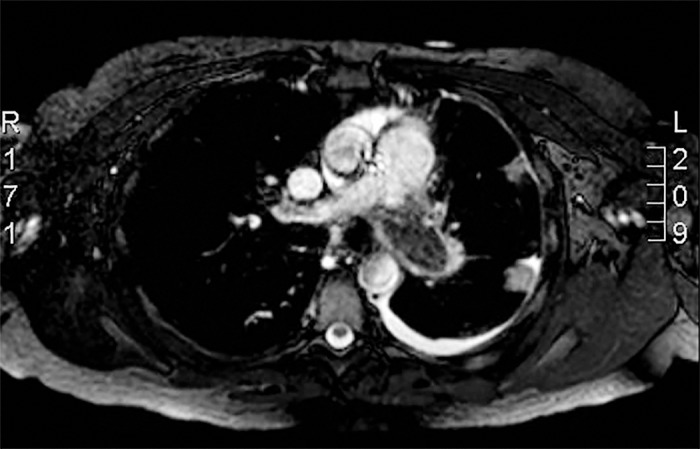
Preoperative cardiac magnetic resonance imaging demonstrating a thrombus occluding the left pulmonary artery.
She was deemed to be a suitable candidate for surgery. A PTE was performed via a median sternotomy incision on cardiopulmonary bypass (CPB) with deep hypothermia and 55 minutes of circulatory arrest. Cannulation for CPB was performed with a standard technique in the ascending aorta with bicaval venous drainage from the superior and inferior vena cava. The patient was cooled to 18°C, and 20-minute periods of circulatory arrest were performed to facilitate exposure and technical precision for the endarterectomies in a bloodless surgical field. The left pulmonary artery was opened first through a longitudinal incision, and an embolectomy of the occlusive thrombus was performed followed by an extensive endarterectomy (Figure 2). The right pulmonary artery was then exposed under the superior vena cava (Figure 3), and an extensive endarterectomy was performed through a longitudinal incision (Figure 4). A third period of circulatory arrest was performed to complete the endarterectomy on the left side. The pulmonary arteries were then closed primarily, and the patient was rewarmed on CPB. After separation from CPB, right ventricular function and the tricuspid valve were examined with transesophageal echocardiography. The right ventricle demonstrated improved function with minimal tricuspid regurgitation. The systolic pulmonary artery pressure decreased from 70 mmHg to 40 mmHg measured directly by the pulmonary artery catheter. The patient did well, required no transfusions, and was discharged on postoperative day 4.
Figure 2.
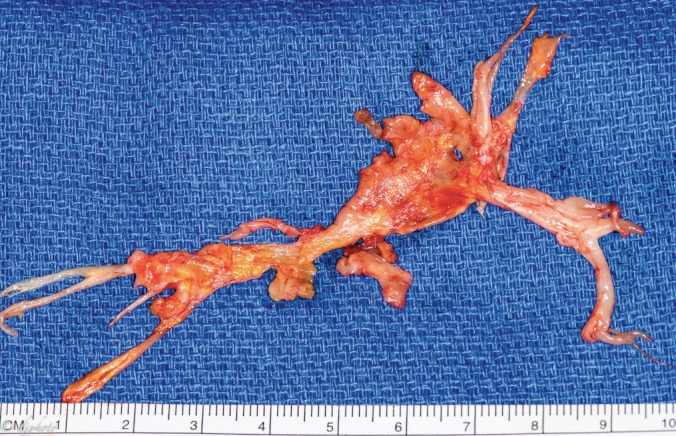
Left endarterectomy surgical specimen. Note the branching pattern from the main pulmonary artery through the subsegmental branches. (A color version of this photograph is available online at www.ochsnerjournal.org/toc/ochs/17/1 in the Focus on Transplantation section.)
Figure 3.
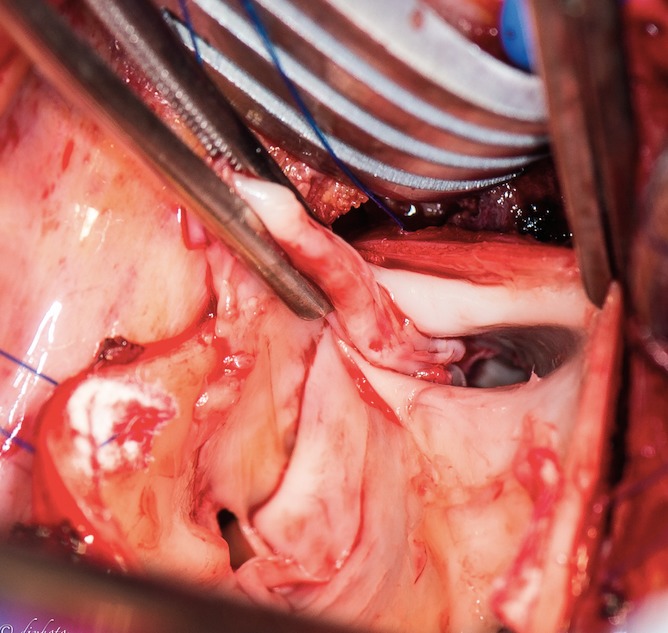
View down the right pulmonary artery during endarterectomy. The artery is opened longitudinally and the segmental branches can be visualized. (A color version of this photograph is available online at www.ochsnerjournal.org/toc/ochs/17/1 in the Focus on Transplantation section.)
Figure 4.
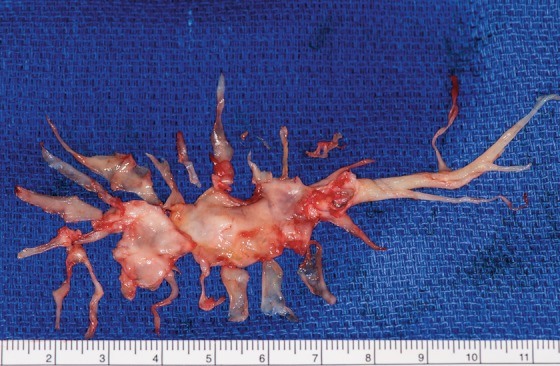
Right endarterectomy surgical specimen starting at the main artery and extending into the subsegmental branches. (A color version of this photograph is available online at www.ochsnerjournal.org/toc/ochs/17/1 in the Focus on Transplantation section.)
At a follow-up visit, the patient reported significant functional improvement. She underwent 1 month of cardiac rehabilitation. She was able to return to work and had no physical limitations. She remained on anticoagulation with the plan to continue it indefinitely. At her 1-month postoperative echocardiogram, the tricuspid regurgitation was mild and has stayed mild with subsequent echocardiograms, as opposed to the severe tricuspid regurgitation seen preoperatively. Her 1-month echocardiogram showed an estimated systolic pulmonary artery pressure of 42 mmHg compared to the 81 mmHg measured by echocardiogram preoperatively.
The patient made significant gains in her postoperative 6-minute walk distance (6MWD). At 1 month, she walked 396 meters; at 3 months, she walked 649 meters; and at 7 months, she walked 670 meters.
Three months postoperatively, her systolic pulmonary artery pressure was estimated to be approximately 35 mmHg. The right heart catheterization performed at 6 months demonstrated an increase in the pulmonary artery pressure of 55/17 mmHg, with a mean pressure of 31 mmHg. The right atrial pressure was normal at 13 mmHg. The echocardiogram performed at this time showed mild tricuspid regurgitation and moderate right ventricular dysfunction with a normal central venous pressure. The patient was started on riociguat for persistent PH. She continues to be followed by the heart failure team.
DISCUSSION
Although successful operations for CTEPH have been performed for more than 50 years, the mainstay of treatment for this disease remains medical, with surgical therapy concentrated in a few high-volume centers. An unknown number of undiagnosed patients and patients who are not referred to a surgeon might have curable disease. Before PTE was developed, the 2-year mortality with CTEPH was >80% when the patient's mean pulmonary artery pressure was >50 mmHg.6 The PTE procedure is well described and replicable, although most studies demonstrate a learning curve for the procedure.6 Overall, 4-year mortality rates after surgery appear to be approximately 15%.9 Mortality rates correlate with the postoperative pulmonary vascular resistance.10
Screening is important for patients with PH because symptoms are often vague and may not manifest for many years. In high-risk populations, such as patients with acute pulmonary embolism who have evidence of right ventricle dysfunction or continue to have significant dyspnea, a repeat echocardiogram at 3-6 months is recommended.2,3 Risk factors for developing CTEPH include multiple episodes of pulmonary embolism, thyroid replacement therapy, a history of a splenectomy or malignancy, a ventriculoatrial shunt, a prothrombotic state, and a previous venous thromboembolism.3
The benefit of PTE on right ventricular remodeling is thought to occur by 3 months, with a further increase in pulmonary function by 12 months postoperatively.8 After surgery, the changes seen on echocardiogram are the resolution of tricuspid regurgitation, a decrease in size of the right ventricle and right atrium, and normalization of the E/A ratio.11 Some studies report additional decreases in mean pulmonary artery pressure by the 12-month mark but no additional improvements beyond 2 years from surgery.5
This case demonstrates the issue of a dysfunctional tricuspid valve that is tempting to fix at the time of the operation. This intervention is not necessary as the valvular issue usually resolves as pressures normalize, as in this case. Of note, the severity of the valvular disease does not necessarily correlate with the degree of PH.
Across different studies at large-volume centers, approximately 35% of patients have residual PH after PTE, although the residual PH does not appear to significantly impact their 5-year survival, especially with adjuvant disease-modifying medications.3 One predictor of residual PH is a low preoperative 6MWD.12 Patients who develop residual PH also are noted to have high preoperative mean pulmonary artery pressures.4 Interestingly, despite our patient's high postoperative pulmonary artery pressures, her 6MWD increased quite dramatically, with an increase of more than 250 meters from her 1-month to her 7-month 6MWD. This increase is higher than the median increase of approximately 100 meters demonstrated in large studies.4,10
Although bilateral lung transplant is the only option for some patients with PH secondary to other etiologies, transplantation should not be the first-line treatment for patients with CTEPH. In the United States in 2011, patients with PH from all causes comprised 6.9% of wait lists (with the minority of these cases of pulmonary artery hypertension being secondary to CTEPH) and 4.4% of lung transplants. The scoring system for the lung transplant wait list does not prioritize patients with PH as it does not account for right ventricle function.13 Prior PTE does not preclude transplant. The International Guidelines for the Selection of Lung Transplant Candidates from 2014 have specific recommendations for indications for transplant for patients with PH, including right atrial pressure >15 mmHg, 6MWD <350 meters, and failure of medical management. Again, it is important to note that previous chest surgery is not a contraindication to lung transplant, although it does increase the risk of bleeding, phrenic nerve injury, need for reexploration, renal dysfunction, and primary graft dysfunction.14
The development of new disease-modifying medications to treat PH secondary to CTEPH is having an impact on the management of these patients. The drug classes include endothelin receptor antagonists (bosentan, ambrisentan, and macitentan), drugs that stimulate the nitric oxide/cyclic guanosine monophosphate pathway (sildenafil, tadalafil, and riociguat), and drugs that enhance the prostacyclin pathway (epoprostenol, treprostinil, and iloprost). Riociguat (Adempas) has been shown to increase 6MWD in patients with persistent pulmonary artery hypertension after PTE, with the most common side effects being hypotension and the need for precautions in women of childbearing age (such as our patient) because of teratogenicity.15 Convincing evidence has not yet been presented showing a benefit from any of these medications in a synergistic way as neoadjuvant treatment prior to surgery or as a bridge to surgery. Instead, they are best utilized in patients with disease that will not benefit from an operation or in patients with residual or recurrent disease after PTE.3
CONCLUSION
This case illustrates the surgical treatment for chronic pulmonary thromboembolic disease causing heart failure and the postoperative course. Taking a multidisciplinary approach to these complicated patients both preoperatively and postoperatively is important. CTEPH is sometimes considered a disease working on 2 levels as in our patient: she had the macroscopic target of the proximal thrombus and also consequent secondary damage to her lungs with extensive small vessel vasculopathy as evidenced by her residual PH. Although our patient had subjective symptomatic relief almost immediately postoperatively, the objective measures also changed and improved. It remains to be seen what objective benefit she may derive from her medical therapy. Recognition of chronic pulmonary thromboembolic disease as the etiology of PH warrants evaluation for surgery.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article. David Fary provided the intraoperative photographs.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1. Pengo V, Lensing AW, Prins MH, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004. May 27; 350 22: 2257- 2264. [DOI] [PubMed] [Google Scholar]
- 2. Wilkens H, Lang I, Behr J, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations of the Cologne Consensus Conference 2011. Int J Cardiol. 2011. December; 154 Suppl 1: S54- S60. 10.1016/S0167-5273(11)70493-4. [DOI] [PubMed] [Google Scholar]
- 3. Marshall PS, Kerr KM, Auger WR. . Chronic thromboembolic pulmonary hypertension. Clin Chest Med. 2013. December; 34 4: 779- 797. 10.1016/j.ccm.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 4. Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008. May 15; 177 10: 1122- 1127. 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 5. D'Armini AM, Cattadori B, Monterosso C, et al. Pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension: hemodynamic characteristics and changes. Eur J of Cardiothorac Surg. 2000. December; 18 6: 696- 701; discussion 701-702. [DOI] [PubMed] [Google Scholar]
- 6. Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003. November; 76 5: 1457- 1462; discussion 1462-1464. [DOI] [PubMed] [Google Scholar]
- 7. Fares WH, Heresi GA. . Chronic thromboembolic pulmonary hypertension: a worldwide view of how far we have come. Lung. 2016. June; 194 3: 483- 485. 10.1007/s00408-016-9863-6. [DOI] [PubMed] [Google Scholar]
- 8. Suntharalingam J, Goldsmith K, Toshner M, et al. Role of NT-proBNP and 6MWD in chronic thromboembolic pulmonary hypertension. Respir Med. 2007. November; 101 11: 2254- 2262. [DOI] [PubMed] [Google Scholar]
- 9. Corsico AG, D'Armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med. 2008. August 15; 178 4: 419- 424. 10.1164/rccm.200801-101OC. [DOI] [PubMed] [Google Scholar]
- 10. Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011. March; 141 3: 702- 710. 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 11. Daniels LB, Krummen DE, Blanchard DG. . Echocardiography in pulmonary vascular disease. Cardiol Clin. 2004. August; 22 3: 383- 399, vi. [DOI] [PubMed] [Google Scholar]
- 12. Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011. February; 141 2: 383- 387. 10.1016/j.jtcvs.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 13. Norfolk SG, Lederer DJ, Tapson VF. . Lung transplantation and atrial septostomy in pulmonary arterial hypertension. Clin Chest Med. 2013. December; 34 4: 857- 865. 10.1016/j.ccm.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 14. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015. January; 34 1: 1- 15. 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 15. Hill NS, Badesch DS, Benza RL, et al. Perspectives on oral pulmonary hypertension therapies recently approved by the U.S. Food and Drug Administration. Ann Am Thorac Soc. 2015. February; 12 2: 269- 273. 10.1513/AnnalsATS.201501-020AS. [DOI] [PubMed] [Google Scholar]


