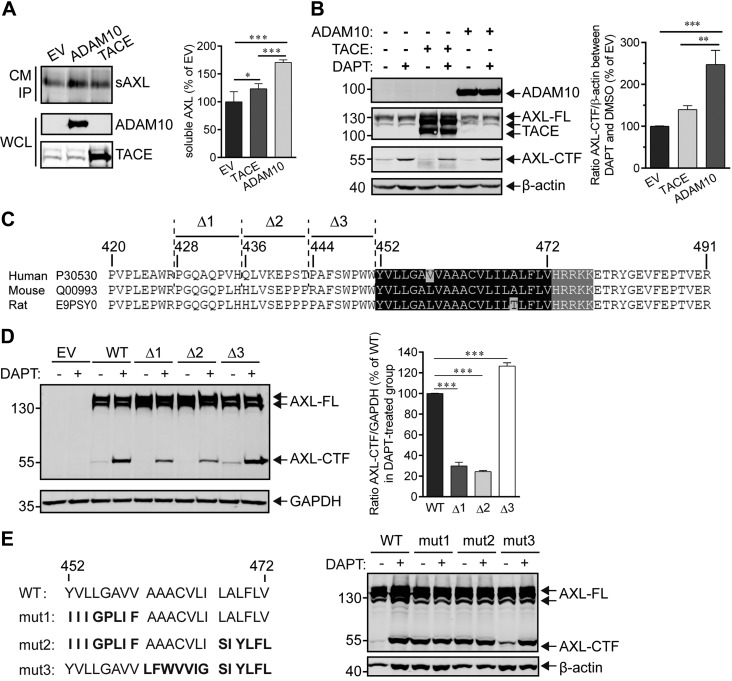
Figure 4.
Determination of α- and γ-secretases cleavage sites in AXL. A) ADAM10 is the major α-secretase responsible for cleavage of the AXL ectodomain. HEK293T cells that stably expressed N- and C-terminal Flag-tagged AXL-FL were transiently transfected with ADAM10 or TACE for 48 h, and the corresponding culture medium (CM) was then collected for immunoprecipitation by using an anti-Flag affinity resin. Proteins bound to the resin were eluted in 2× sample buffer and subjected to Western blot analysis for detection of sAXL (anti-Flag Ab), ADAM10 (anti-Myc Ab), or TACE (anti-Flag Ab). The densitometric estimation of sAXL was performed by using ImageJ software, and levels of sAXL are expressed as a percentage of the empty vector (EV) control group. Data are presented as means ± sem (n = 5). Significance value was estimated using 1-way ANOVA with Bonferroni's multiple comparisons test. *P < 0.05, ***P < 0.001. B) HEK293T cells expressing the Flag-tagged AXL-FL were transiently transfected with ADAM10, TACE, or EV for 24 h. Cells were then incubated for 24 h with DMSO (control) or DAPT to induce AXL-CTF accumulation, which reflects the shedding activity of α-secretase on AXL, and cell lysates were collected and subjected to Western blot to analyze AXL-CTF (anti-Flag Ab), mTACE-Flag (anti-Flag Ab), or ADAM10 (Myc Ab) levels. The relative levels of AXL-CTF were quantified by ImageJ software and the ratios between the DAPT- and DMSO-treated groups were expressed as the percentage of the EV group. Data are presented as means ± sem (n = 4). **P < 0.01, ***P < 0.001, 1-way ANOVA with Bonferroni's multiple comparisons test. C) Amino acid sequence alignment of the juxtamembrane domains of human, mouse, and rat AXL. Δ1, Δ1, or Δ3 refers to the deleted regions in hAXL mutants. The black shaded region is the TMD, and the gray area is the conserved basic amino acid domain in the cytoplasmic region. D) Putative AXL cleavage sites in α-secretase. HEK293T cells transiently expressing WT or mutant AXL [i.e., Δ1 (428PGQAQPVH435-deletion), Δ2 (436QLVKEPST443-deletion), or Δ3 (444PAFSWPWW451-deletion)] were treated with DAPT at 10 μM, and cell lysates were analyzed for AXL-FL and AXL-CTF (anti-Flag). AXL-CTF or GAPDH bands in the blot (left) were quantified by using ImageJ software. The ratio between AXL-CTF and GAPDH was calculated and normalized to the WT group (right). Results are expressed as means ± sem (n = 4). ***P < 0.001, 1-way ANOVA with Bonferroni's multiple comparisons test. E) Putative γ-secretase cleavage sites in AXL. HEK293T cells were transiently transfected with WT or mutants AXL (mut1-3; left), in which the TMD was substituted with sequences of the InsR (bold fonts; left), which has previously been used to identify the cleavage sites for γ-secretase (37). Cells were then treated overnight with 10 μM DAPT before analysis of AXL-FL and -CTF (anti-Flag; right). The immunoblot shows representative results of at least 2 independent experiments. β-Actin or GAPDH serve as the loading controls.