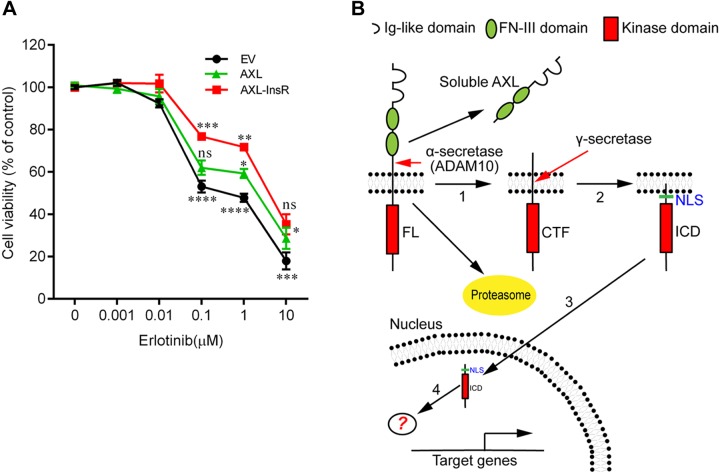
Figure 7.
Ablation of intramembrane cleavage of AXL-FL elevates drug resistance in HCC827 cells. A) Blockage of AXL cleavage mediated by γ-secretase promotes erlotinib chemoresistance in HCC827 cells. HCC827 cells stably expressing WT AXL-FL (AXL-WT) or the γ-secretase–uncleavable AXL mutant (AXL-InsR), or transfected with the empty vector (EV), were incubated for 72 h with the indicated concentrations of erlotinib, and the cell number was measured by using the CellTiter96 Aqueous One Solution Cell Proliferation Assay. Experiments were independently repeated 2× in sextuples, and 1 representative result is shown. Means at each concentration are given as percentage of the DMSO control. Data are presented as means ± sem (n = 6). Statistical analyses were performed by 2-way ANOVA with Bonferroni post-tests. The significance of AXL-InsR compared with AXL-WT is indicated above the red line, whereas the comparison with EV is indicated below the black line, and the significance of AXL-WT and EV is above the green line. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. B) Model for the successive proteolytic processing of AXL by α- and γ-secretases. AXL RTK is sequentially cleaved by α-secretase (1) and γ-secretase (2). Next, AXL-ICD, the cleavage product of the γ-secretase–dependent regulated intramembrane proteolysis, translocates into the nucleus via an NLS (3). By interacting with yet unknown intranuclear binding partners (4), AXL-ICD potentially modulates the expression of genes (for example, NF-κB–dependent genes) implicated in TAM-dependent signaling pathways. In addition, AXL-FL could be processed via the ubiquitin-proteasome proteolytic pathway (51), inhibition of which could accelerate the secretases mediated proteolytic pathway of AXL (1, 2). Ns, no significance.