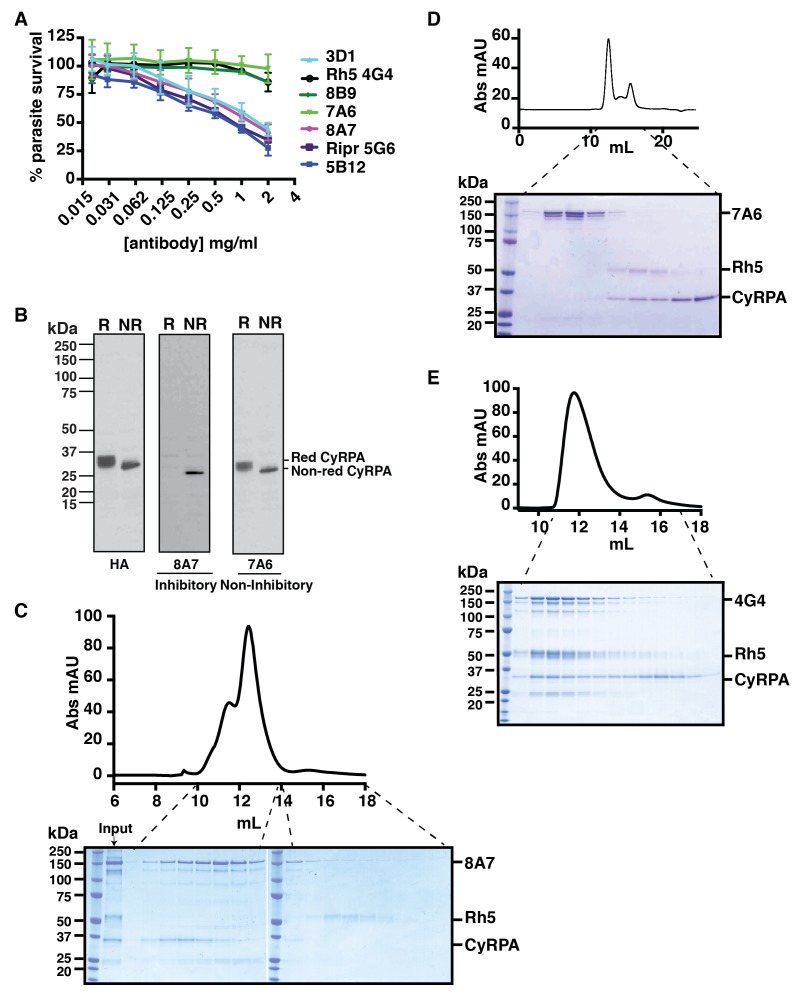
Figure 2. Anti-CyRPA monoclonal antibodies inhibit P. falciparum growth and interaction of PfRh5.
(A) In vitro growth inhibition (GIA) assays were performed to assess the abilities of the monoclonal antibodies raised against recombinant CyRPA to block P. falciparum parasite growth in human erythrocytes. Assays were performed twice in triplicate and error bars denote SD of the mean of 6 values. Anti-Rh5 mAb 4G4 (not inhibitory) and anti-Ripr mAb 5G6 (inhibitory) are included as GIA controls. (B) Immunoblot of inhibitory (8A7) and non-inhibitory (7A6) monoclonal antibodies against proteins from CyRPA-HA tagged transgenic P. falciparum schizonts in reduced (R) and non-reduced (NR) condition. The P. falciparum parasites expressed haemagglutinin-tagged CyRPA protein. (C) Monoclonal antibody 8A7 blocked binding of PfRh5 to CyRPA. PfRh5 and CyRPA were incubated together with 8A7 and the complex formation monitored by size exclusion chromatography and SDS-PAGE analysis under non-reducing conditions. Monoclonal antibody 8A7 bound to CyRPA, preventing the PfRh5/CyRPA complex formation. CyRPA was co-eluted with 8A7 in the earlier fractions and the free Rh5 eluted in the later fractions. (D) Monoclonal antibody 7A6 did not inhibit the formation of the PfRh5/CyRPA complex as monitored by size exclusion chromatography and SDS-PAGE analysis. This antibody did not bind to native CyRPA indicating the linear epitope is not surface exposed. (E) Non-inhibitory anti-PfRh5 monoclonal antibody 4G4 did not significantly inhibit the formation of the PfRh5/CyRPA complex. The PfRh5/CyRPA complex bound to antibody 4G4 and was eluted together with 4G4 as a tri-molecule complex in the earlier fractions.