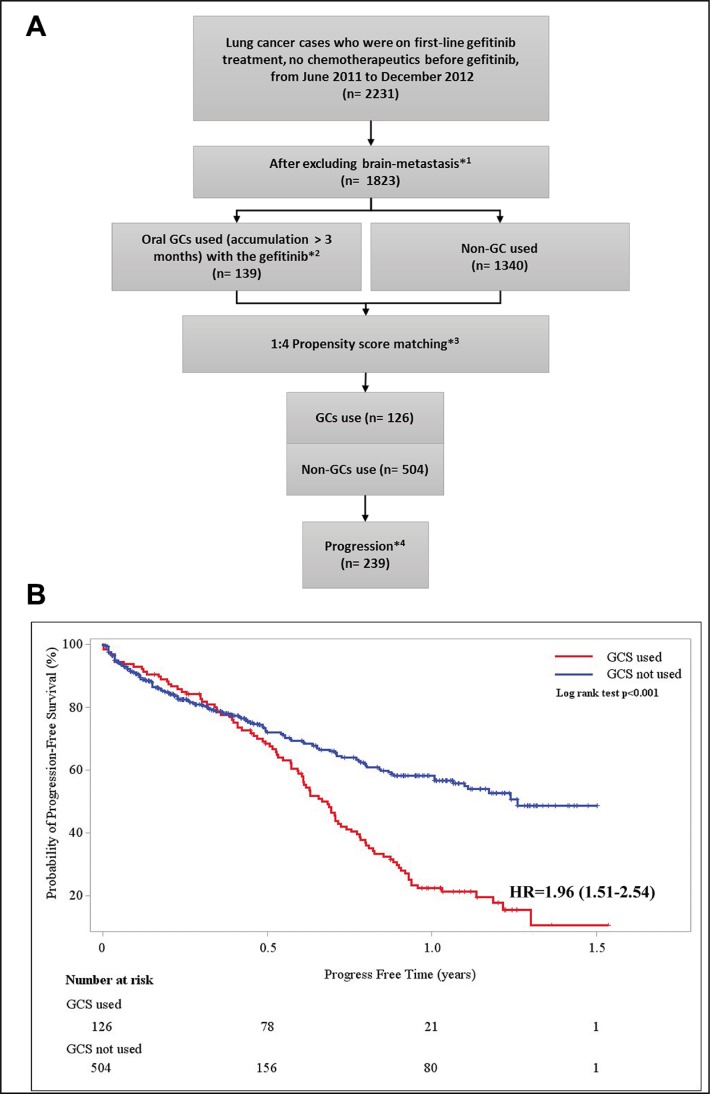
Figure 4. Population data analysis scheme and progression free survivals (years) for patients using gefitinib only or in combination with GCs.
(A) Flow scheme for analyzing the effects of GCs comedication using the Taiwan NHI claim database. *1Brain-metastasis (ICD-9-CM 198.3) was excluded because use of GCs was recommended management for such disease status. *2A group of cases (n = 139) who received oral GCs (dexamethasone, methylprednisolone or prednisolone) for more than 3 months during gefitinib therapy was identified. *3Propensity score was calculated using gender and age, as well as disease status including diabetes, hypertensive heart disease, kidney disease, respiratory conditions (including chronic obstructive pulmonary disease, asthma, and bronchitis), metastasis and endotracheal intubation. *4 Due to the nature of the NHI claim database, in this observational study, patients with progression was defined as: prescription of a new chemotherapeutic drugs or death. (B) Kaplan-Meier analysis of probability of progression-free in gefitinib alone or concomitant use with oral GCs during gefitinib therapy. HR shows the hazard ratio and the 95% confidence intervals (CIs) of the GCs comedication group compared to the non-GCs used group.