Figure 4. Both cell context-specific physiological endogenous RA synthesis and transcriptional functionality of RARA differentially determine breast cancer cell fate.
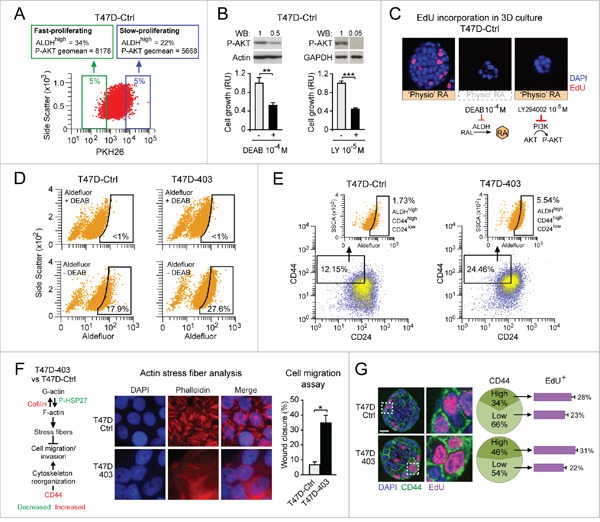
A. Cytofluorimetric analysis of PKH26-labeled T47DCtrl cells shows that the fast-proliferating cell subset (green frame) contains more cells with high ALDH activity (ALDHhigh cells, detected by Aldefluor staining) and expresses more P-AKT relative to the slow-proliferating cell subset (blue frame). B. Inhibition of either ALDH-mediated RA synthesis with DEAB (left), or PI3K activity with LY294002 (right) results in a decrease of both P-AKT level (top) and cell proliferation (bottom) (**p<0.01, ***p<0.001). C. Treatment with either DEAB or LY294002 inhibits T47DCtrl proliferation also in 3D culture. D-E. Increased inhibition of RARA transcriptional function in T47D403 cells leads to expansion of both ALDHhigh (D) and ALDHhigh/CD44high/CD24low (E) subpopulations relative to T47DCtrl. F. Global gene/protein expression analyses highlight pro-invasive molecular changes in T47D403 vs. T47DCtrl (left). Consistently, T47D403 cells show defective phalloidin-stained actin stress fibers (middle) and increased cell migration in the wound healing assay (right) (*p<0.05). G. Confocal analysis shows that 3D T47D403 acini have a higher proportion of CD44high cells relative to 3D T47DCtrl acini (**p<0.01), and that the T47D403 CD44high cell subpopulation contains more proliferating (EdU-positive) cells than the CD44low cell subpopulation (**p<0.01). Significance calculated by Student's t-test.
