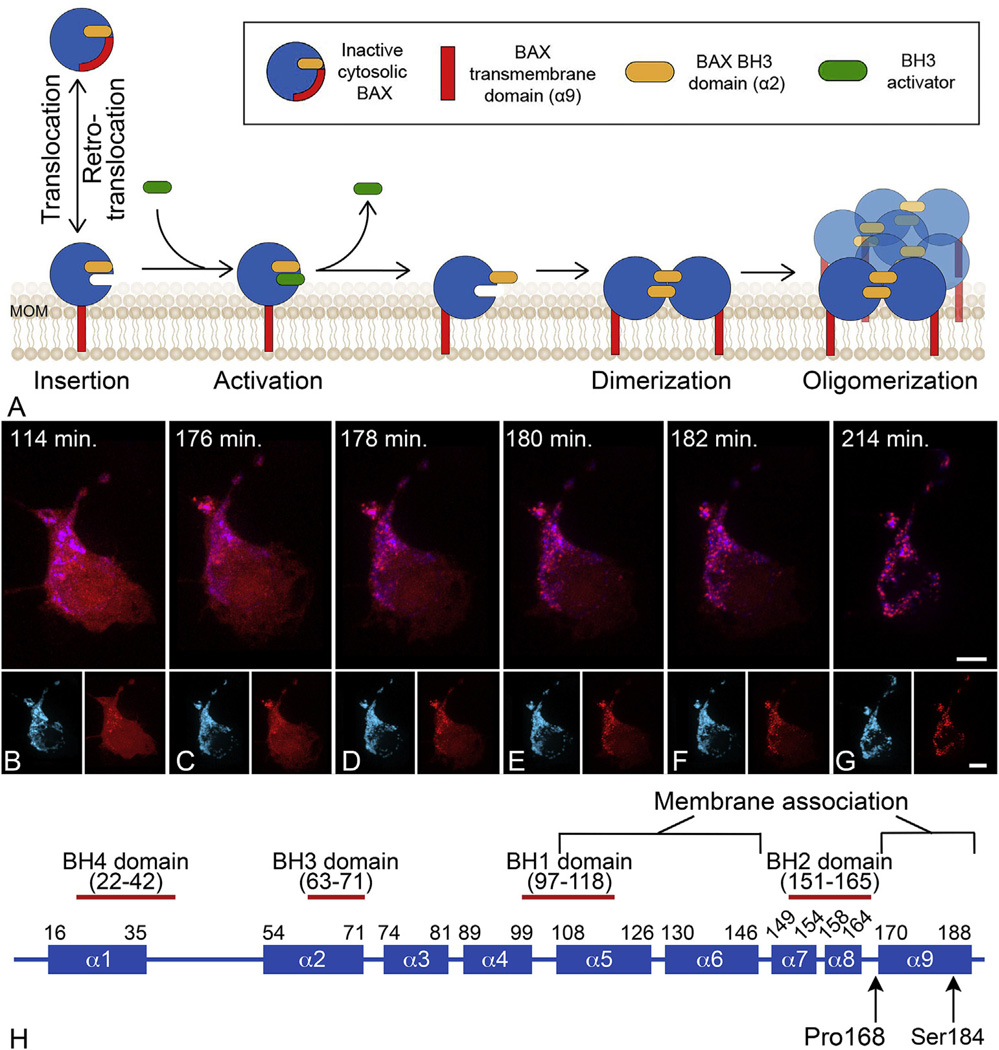
Fig. 1.
BAX protein activation at the mitochondrial outer membrane (MOM). (A) Schematic of the stages of BAX protein activation. Latent BAX proteins are globular and predominantly cytosolic. In healthy cells, globular BAX is in equilibrium with the MOM, and is retrotranslocated to the cytosol by BCL-X It is expected that the interaction with the MOM involves exposure of the α9 helical domain. Early during apoptosis, the cell expresses BH3-only proteins that have the function of sequestering anti-apoptotic proteins (not shown in diagram) and interacting with membrane associated BAX to cause further conformational changes to expose both a hydrophobic groove and the BH3 domain of BAX. This change allows homodimerization of BAX which then nucleates the formation of a large molecular weight oligomer. It is not clear at what stage activated BAX begins to form pores, but there is evidence that proteolipidic pores large enough to allow the release of cytochrome c can be formed by dimers, while oligomers can form supramolecular pores. Modified from (Czabotar et al., 2014). (B–G) Time lapse photography of a D407 tissue culture cell undergoing the process of BAX translocation and oligomerization to the mitochondria. Mitochondria are identified by blue fluorescent protein fused with a mitochondria localization peptide. BAX is identified by fusion to the mCherry fluorescent protein. The time stamp indicates the elapsed time since the addition of staurosporine to induce apoptosis. In these cells, BAX activation and completion to the large molecular weight oligomer occurs within 20 min. During this process, BAX moves from a diffuse cytoplasmic localization to a bright punctate appearance coincident with the mitochondria. The small insets for each frame show the individual channels for imaging. Size bars = 5 µm. (H) Domain map of mammalian BAX showing the 9 different α-helices. The numbering refers to the amino acid sequence. Two amino acids that affect BAX function are indicated (see text).