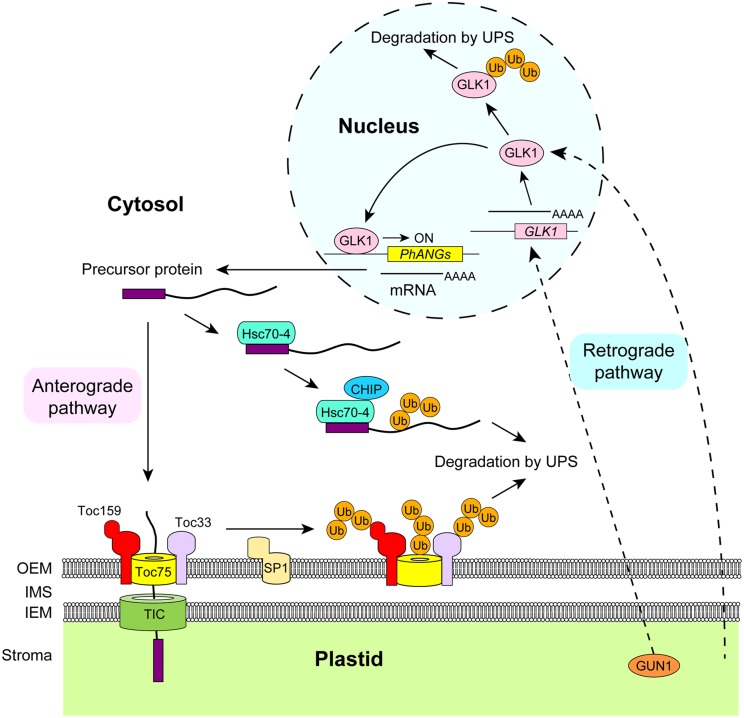
FIGURE 1.
Control of bidirectional signaling between plastids and the nucleus by the ubiquitin–proteasome system. Chloroplast development is promoted by the expression of nuclear-encoded PhANGs and the import of their products into chloroplasts. When excess precursors are produced, they are recognized by the heat shock cognate 70-4 (Hsc70-4) complex in the cytosol. Subsequently, they are polyubiquitinated by an E3 ubiquitin ligase, carboxy terminus of Hsc70-interacting protein (CHIP), resulting in their degradation by the proteasome. The translocon at the outer envelope membrane of chloroplasts (TOC) complex is also directly targeted by the ubiquitin proteasome system. At least three TOC components, Toc159, Toc75, and Toc33, are polyubiquitinated by a membrane-anchored E3 ubiquitin ligase, suppressor of ppi1 locus1 (SP1). To further optimize the amount of protein import into chloroplasts, retrograde signals from chloroplasts regulate the level of the GOLDEN2-LIKE 1 (GLK1) transcription factor in the nucleus. Polyubiquitination of GLK1 is induced when chloroplast biogenesis is inhibited. The degradation of GLK1 results in the down-regulation of PhANGs, thereby preventing the accumulation of unnecessary precursor proteins in the cytosol. GLK1 is also regulated by retrograde signals at transcriptional level, and this regulation is mediated by GENOMES UNCOUPLED 1 (GUN1). Although this figure proposes a model for photosynthetic tissues, similar regulation by the ubiquitin–proteasome system appears to play key roles in plastid development in other tissues. Note that a number of other pathways between plastids and the nucleus have been identified, and those pathways are not shown in this figure due to space limitations but can be found in other adequate reviews. UPS, ubiquitin–proteasome system; OEM, outer envelope membrane; IEM, inner envelope membrane; IMS, intermembrane space; Ub, ubiquitin; PhANGs, photosynthesis-associated nuclear genes.