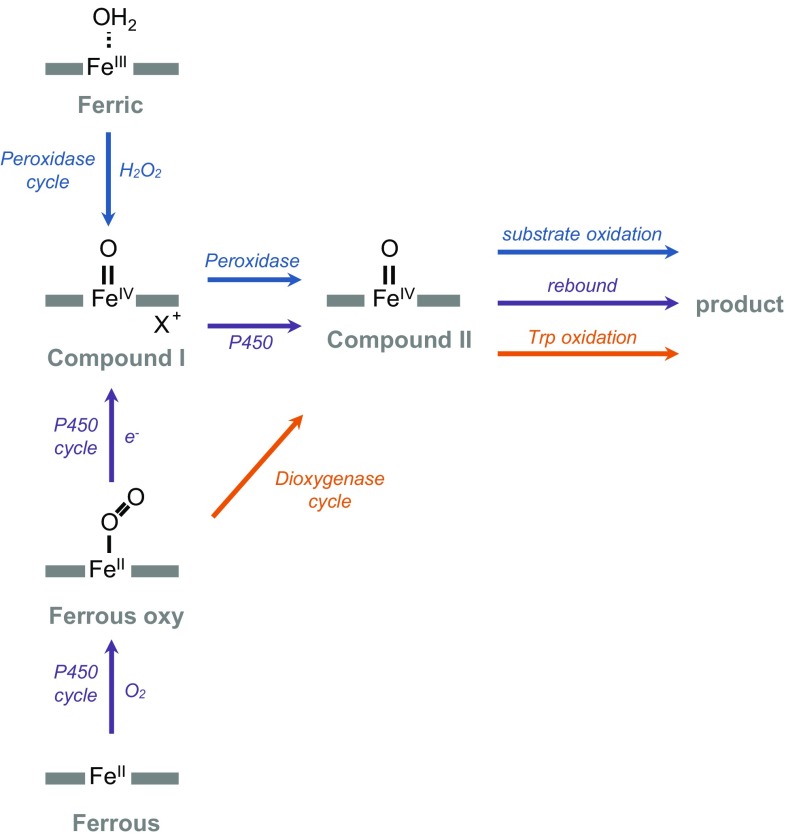
Fig. 7.
A comparison of mechanisms of oxygen activation in different heme enzymes. The well-known peroxidase mechanism (blue arrows) goes via ferric heme directly to Compound I and then to Compound II by one electron oxidation of substrate [114]. The P450s (purple arrows) use the same Compound I species but they access it through the ferrous oxy species by one electron reduction, and by rebound mechanisms access the same Compound II species [115, 116]. The identification [97, 101, 102] of a Compound II species in IDO (which accumulates in the steady state) aligns the dioxygenase mechanism (orange arrows) with these established patterns of reactivity in other heme systems. It has been assumed that IDO and TDO react by the same mechanism, but Compound II in TDO has never been detected in the steady state. There is evidence that the absence of Compound II in the steady state in TDO might be due to a change in the rate-limiting step in TDO compared to IDO, such that Compound II does not accumulate [117]. Note that there is also evidence [118] that IDO can exhibit indole peroxygenase activity (i.e. a peroxide-dependent insertion of oxygen into indole), similar to the well-known peroxide shunt of the P450s