Fig. 7.
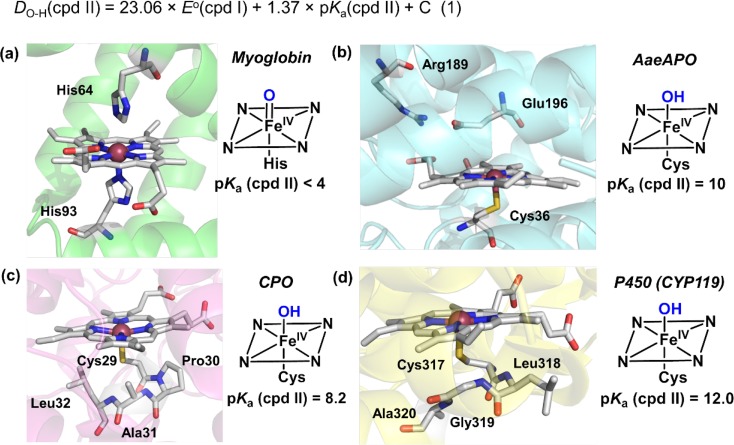
Equation 1 shows the Bordwell equation that relates the newly formed FeO–H bond energy to its pK a and redox potential via a Hess cycle. C is a constant depending on the solvent and the electrode. For aqueous solution and normal hydrogen electrode, the value of C is 57.6 kcal/mol. Figure 2a–d shows the active site structures and pK as of compound II for common heme-containing proteins. Active site structures were rendered using following structures: a myoglobin (PDB: 2V1H); b aromatic peroxygenase from Agrocybe aegerita (AaeAPO, PDB: 2YOR); c chloroperoxidase (CPO, PDB: 2J19); d cytochrome P450 (CYP119, PDB: 1IO7). Colors: iron (dark pink), nitrogen (blue), oxygen (red), carbon (silver), sulfur (yellow)