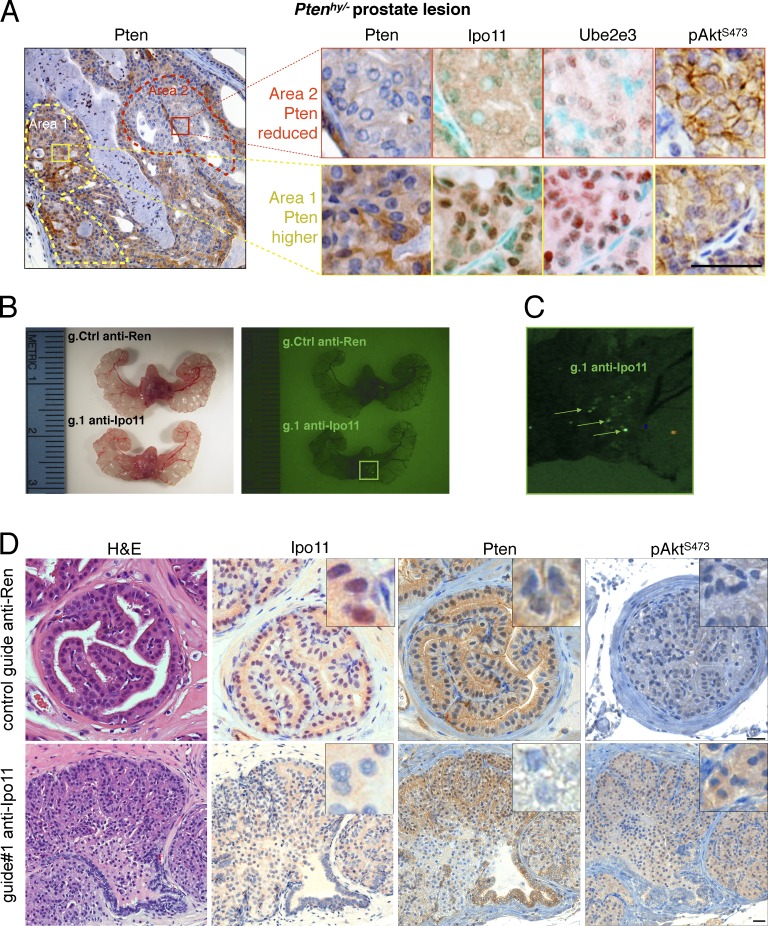
Figure 6.
Importin-11 loss causes prostate neoplasia. (A, left) Typical Pten IHC of prostate from a Ptenhy/− mouse displays spontaneous reduction of Pten protein in area 2 (red dashes) compared with area 1 (yellow dashes). (right) Comparative IHC of Pten, Ipo11, Ube2e3, and p-AktS473 staining in area 2 (top) and area 1 (bottom) confirms correlation between Pten suppression and Ipo11 loss/malfunction as scored by Ube2e3 mislocalization. Note that methyl green (instead of hematoxylin) was used as nuclear counterstain in Ipo11 and Ube2e3 staining to reveal the faint remaining nuclear staining of Ipo11 and Ube2e3. Bar, 50 µm. (B) Comparison of mouse prostates 8 wk postinjection with lentiviral plasmids carrying Cas9, Venus (for fluorescence); control guide: anti-Ren (g.208); or guide targeting Ipo11: anti-Ipo11 (g.1) under light (left) and fluorescence (right). Fluorescence signals are not seen in anti-Ren–injected (g.208) animals. (C) Zoomed fluorescence picture of anti-Ipo11 injections show green signals (arrows) pointing to the sites at which virus injections led to the expression of Venus and genome-editing events. (D) IHC of prostate from lentivirus Cas9-CRISPR–injected mice shows neoplastic lesions with loss of epithelial architecture that was absent from Renilla control-targeted prostates. IHC analysis confirmed efficient Ipo11 suppression in anti-Ipo11–injected (bottom) but not anti-Ren–injected prostate (top). Comparative IHC of Pten, staining in anti-Ren control (top) and anti-Ipo11 tissue (bottom) confirms correlation between Pten suppression in regions with loss of nuclear Ipo11 and increased p-Akt tissue levels. Bars, 50 µm.