Figure 6. Hsp70 remains associated with the assembly following sHsp displacement.
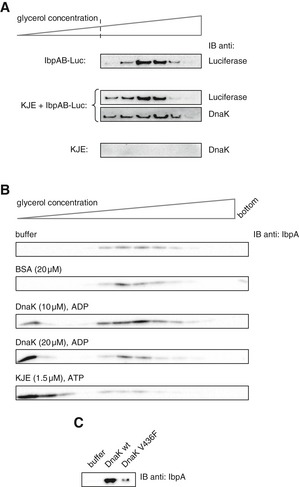
- Purified IbpAB–luciferase assemblies were incubated with buffer or KJE and 2 mM ATP at 25°C for 60 min and resubjected to glycerol gradient sedimentation. Fractions were collected from the top of the gradient, and luciferase and DnaK were visualized by Western blot analysis. To avoid visualizing dominant, unbound DnaK, only the lower, IbpAB–luciferase assembly‐containing fractions of the gradient are shown. Sedimentation analysis of KJE only is provided as a control. IbpAB–luciferase assemblies (approximate stoichiometry 1:0.8:1), DnaK, DnaJ and GrpE were present at 100 nM (calculated for luciferase monomer), 1 μM, 0.3 μM and 0.3 μM concentrations, respectively.
- DnaK binding to IbpAB–luciferase assemblies is sufficient to release IbpAB. Purified IbpAB–luciferase assemblies (100 nM) were incubated with the indicated components for 60 min and subjected to glycerol gradient sedimentation. Fractions were collected from the top of the gradient and IbpA was visualized by Western blot following SDS–PAGE.
- Purified IbpAB–luciferase assemblies (100 nM) were incubated for 60 min with buffer or DnaK (10 μM) or DnaK V436F (10 μM) and subjected to glycerol gradient sedimentation as in Fig 4B, but instead of showing the entire gradients, the fractions corresponding to the released material (1–3) were pooled together and IbpA was visualized by Western blot following SDS–PAGE.
Source data are available online for this figure.
