Figure 9. Schematic model of the Hsp100‐Hsp70 bichaperone system action on sHsp–substrate assemblies.
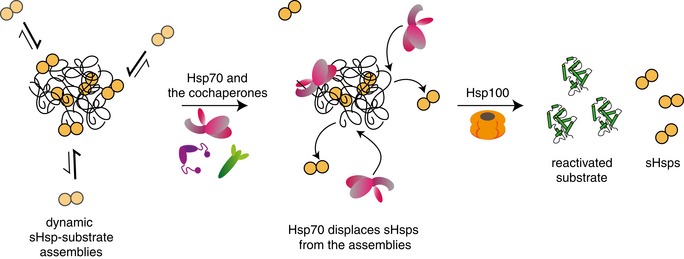
sHsp–substrate assemblies consist of a stable sHsp–substrate core and an dynamic outer shell, comprising the majority of sHsps. Surface‐exposed sHsps are dynamically binding and dissociating from the sHsp–substrate assemblies, with the equilibrium shifted towards the bound state. Hsp70 outcompetes sHsps in binding to the assembly surface, protecting the sHsp–substrate core and rendering sHsps in the unbound state. Once the sHsps are displaced from the surface, the misfolded polypeptides are extracted from the assemblies and refolded by a concerted action of the Hsp100‐Hsp70 bichaperone system.
