Figure 3. Interaction between syntaxin‐1 and Munc13 is disrupted by the syntaxin‐1 RIAA (R151A, I155A) and Munc13‐1 NFAA (N1128A, F1131A) mutations.
-
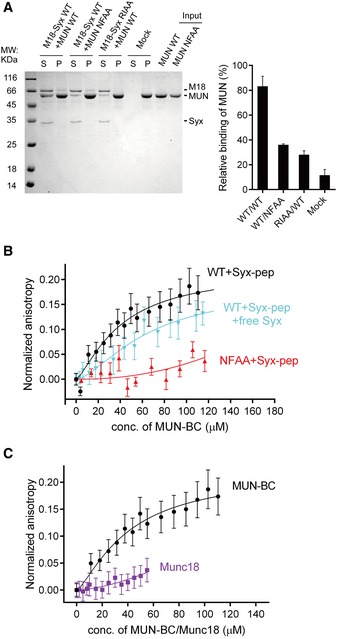
ACo‐flotation assay detecting the interaction between the MUN domain and proteoliposomes that reconstituted the Munc18‐1/syntaxin‐1 complex (left panel). Quantification of the results is shown in the right panel. “Mock” refers to the co‐flotation experiment of the MUN domain and plain (protein‐free) liposomes. S, supernatant; P, pellet. A representative Coomassie brilliant blue‐stained electrophoresis gel from one of three independent experiments is shown. Data were processed by ImageJ (NIH) and shown as mean ± SD for n = 3 technical replicates.
-
B, CInteraction of the fluorescent dye‐labeled syntaxin‐1 peptide (residues 148–162, referred to as Syx‐pep) with the MUN‐BC fragment (with or without the cytoplasmic region of syntaxin‐1, residues 2–253, referred to as free Syx), its NFAA mutant (B), and Munc18‐1 (C) as monitored by ensemble fluorescence anisotropy measurements. Nonlinear curve fits were performed by using the Hill equation without constraints. Shown are means ± SEM (n = 10).
