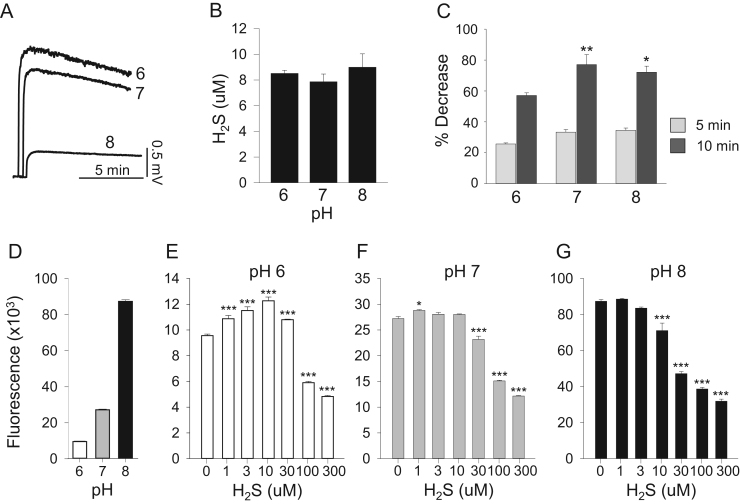
Fig. 4.
Effect of pH on catalase-mediated H2S oxidation measured amperometrically (A-C) and on interactions with DCF oxidation (D–G). (A) Sensor response to 10 μM H2S standard (output in mV) decreases as pH increases due to pH-dependent decrease in dissolved H2S gas. (B) Sensor response to 10 μM H2S in presence of 25 μM catalase corrected for pH effect on dissolved H2S. Peak H2S is similar after correction but less than 10 μM due to initial H2S consumption. (C) Percent H2S consumption by 25 μM catalase at 5 and 10 min after H2S injection. D. Catalase-mediated oxidation of 10 μM DCF increased over nine-fold as pH was increased from 6.0 to 8.0. E-G. Effect of H2S on catalase-mediated DCF oxidation at pH 6.0, 7.0 and 8.0. At pH 6.0, low H2S concentrations increased DCF oxidation and higher concentrations inhibited it. As pH increased, the stimulatory effect was lost and H2S became solely inhibitory. Mean±SE (n=3, B, C; n=4, D-G); *, p<0.5; **, p<0.01; ***, p<0.001.