Figure 5.
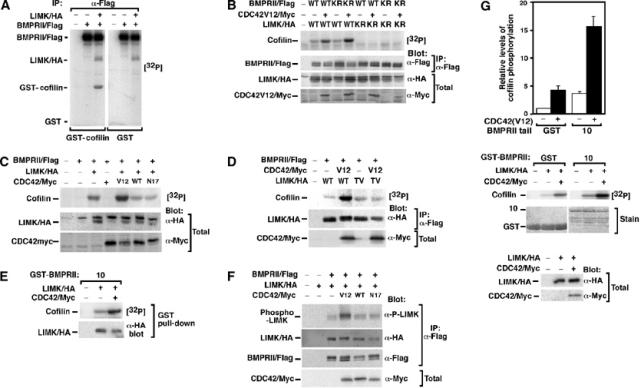
BMPRII-bound LIMK1 synergizes with Cdc42 to phosphorylate cofilin. (A–C) Lysates from transiently transfected COS-1 cells were immunoprecipitated with anti-Flag antibodies and the activity of associated LIMK1 was determined using an in vitro kinase assay with GST-cofilin as substrate. (A) BMPRII-bound LIMK1 phosphorylates cofilin. (B) The kinase activity of LIMK1 but not BMPRII is required for phosphorylation of cofilin. (C) Coexpression of constitutively active (V12) but not wild-type (WT) or dominant-negative (N17) Cdc42 enhances BMPRII-bound LIMK1 activity. (D) Cdc42-dependent enhancement of LIMK1 is lost in an LIMK1 mutated in the activation site residue, Thr508 (LIMK TV). (E) LIMK1 coexpressed with or without Cdc42(V12) in transiently transfected COS-1 cells was isolated using the GST-BMPRII tail construct 10 and the ability of bound LIMK1 to phosphorylate cofilin was determined using an in vitro kinase assay. (F) Lysates from transiently transfected COS-1 cells were immunoprecipitated with anti-Flag antibodies and activation of associated LIMK1 was determined by anti-phospho-LIMK immunoblotting. (G) LIMK1, immunoprecipitated from transiently transfected COS-1 cells, was separated into two equal aliquots and then was incubated for 60 min with bacterially expressed BMPRII tail fragment or GST alone prior to an in vitro kinase assay. Phosphorylation of cofilin was quantitated (top panel) in duplicate from a representative experiment. Protein levels were confirmed by Coomassie staining and immunoblotting (middle and bottom panels).
