Figure 6.
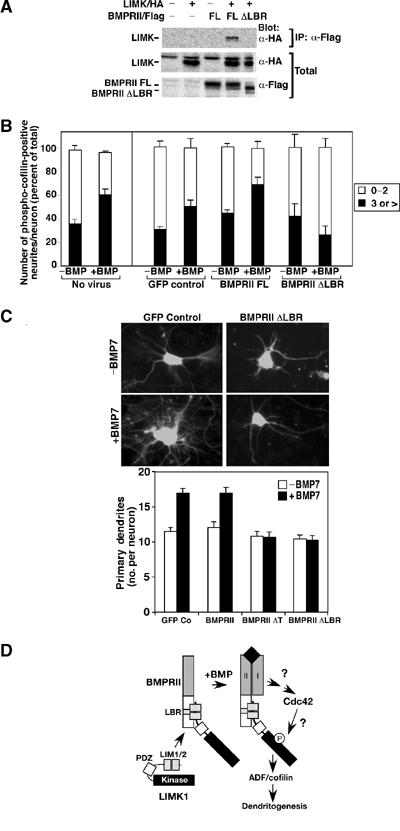
Disruption of LIMK1 interaction with BMPRII blocks cofilin phosphorylation and dendritogenesis. (A) Cell lysates from COS-1 cells transiently transfected with full-length BMPRII or a version lacking the LIMK1 binding region (BMPRII ΔLBR) were subjected to anti-Flag immunoprecipitation and associated LIMK1 was detected by anti-HA immunoblotting. (B) Primary cortical neurons were infected with the indicated adenoviral constructs and the number of phospho-cofilin-positive dendrites per neuron was determined as in Figure 2C. Shown is the mean±s.e.m. for three independent experiments with a minimum of 20 neurons/condition determined in each experiment (right). For direct comparison, the results obtained using noninfected neurons (from Figure 2C) are shown on the left. (C) The number of dendrites/neuron in primary cortical neurons was determined as in Figure 1A. Immunofluorescent images of GFP-containing neurons (top) and quantitation of a representative experiment are shown (bottom). (D) A model for BMP-dependent regulation of dendrite formation. BMP-induced activation of Cdc42 cooperates with binding of LIMK1 to the BMPRII tail to provide for high levels of LIMK1 activity. The mechanism of BMP receptor-induced activation of Cdc42 and the pathway leading from Cdc42 to LIMK1 are not known.
