Figure 3.
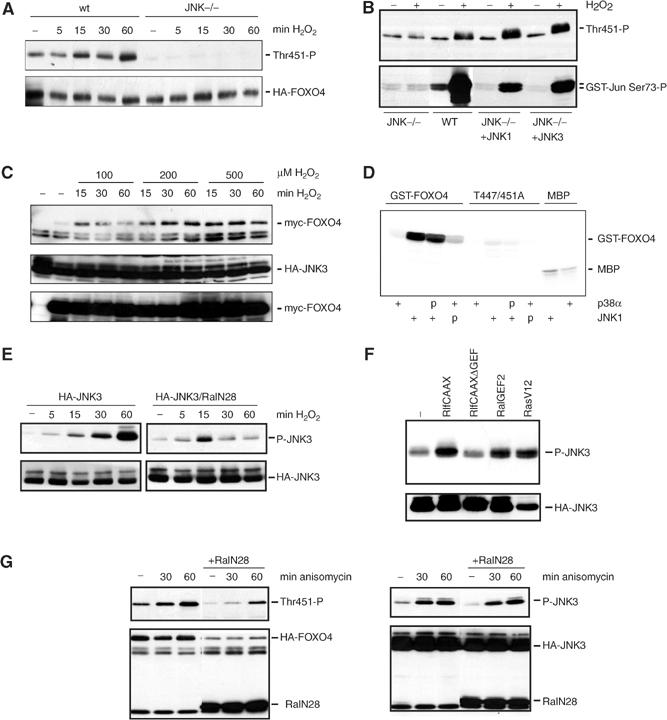
JNK is involved in the H2O2-induced Ral-mediated phosphorylation of T451 and T447 on FOXO4. (A) JNK1,2−/− MEFs, transfected with HA-FOXO4 together with JNK1, JNK3 or an empty vector, were treated with 100 μM H2O2 for the indicated time, and T451 phosphorylation was analyzed on Western blot. wt MEFs were included as control. Similar results were obtained using 200 or 400 μM H2O2. (B) JNK1,2−/− MEFs, wt MEFs and JNK−/− cotransfected with either JNK1 or JNK3, transfected with HA-FOXO4, were left untreated or treated with 100 μM of H2O2 for 60 min. T451 phosphorylation was analyzed. In parallel, a GST-Jun pull-down was performed to measure JNK activity (lower panel). Same results were obtained using 200 or 400 μM H2O2. (C) 293T, transfected with myc-FOXO4 and HA-JNK3, were treated with different concentrations of H2O2 for indicated times. HA-JNK3 was immunoprecipitated and binding of myc-FOXO4 to HA-JNK3 was analyzed on Western blot (upper panel). The lower panels show expression of the constructs. (D) Purified bacterially expressed GST-FOXO4(C) and GST-FOXO4-T447/451A(C) were incubated in the presence (+) or absence of active JNK or active p38α. P indicates pretreatment with either active JNK or p38α in the presence of unlabelled rATP. Prephosphorylation by JNK or p38α did not enhance the ability of p38α or JNK to subsequently phosphorylate GST-FOXO4. MBP substrate was included as control for the activity of active JNK and p38α. (E) 293T cells transfected with HA-JNK3 with or without HA-RalN28 were treated with 100 μM of H2O2 for increasing periods of time. JNK phosphorylation was analyzed on western blot. Similar results were obtained using 200 or 400 μM of H2O2. (F) 293T cells were transfected with HA-JNK3 together with indicated constructs. JNK phosphorylation was analyzed on Western blot. (G) 293T cells transfected with HA-FOXO4 or HA-JNK3 with or without HA-RalN28 were untreated or treated with 10 μg/ml anisomycin for 30 or 60 min. FOXO4-T451 and JNK phosphorylations were analyzed on Western blot. The 60 min treatment of anisomycin induced a three-fold increase in FOXO4-T451 phosphorylation, both in the presence and absence of RalN28.
