Abstract
Background
At present, the expression of MOR1 and its function in gastric cancer remains unclear with evidence suggesting that it is to be involved in tumor progression and metastasis. The study was to assess the clinicopathologic relevance and prognostic value of MOR1 expression in gastric cancer.
Methods
Real‐time quantitative RT‐PCR and immunohistochemical staining were used to detect MOR1 expression in primary gastric cancerous surgical specimens and adjacent nontumorous tissues.
Results
High MOR1 expression was detected in cancerous tumor compared with their adjacent nontumorous tissues. In addition, the chi‐square test revealed that high MOR1 expression was significantly correlated with depth of invasion (p = 0.006), lymph node metastasis (p = 0.001), distant metastasis (p = 0.017), and TNM staging (p = 0.027). Moreover, Kaplan–Meier analysis revealed a significant association between MOR1 expression and overall survival. High expression of MOR1 was identified as an independent and significant predictor gene of reduced postoperative survival.
Conclusion
We conclude that MOR1 expression may be a useful biomarker for better prediction of the clinical outcome and management of gastric cancer patients.
Keywords: MOR1, gastric cancer, prognosis, metastasis
Introduction
Gastric cancer (GC) ranks fourth in incidence and second in mortality among all cancers worldwide.1 Despite the decrease in incidence in some regions of the world, GC continues to present a major clinical challenge due to most cases being diagnosed in advanced stages with poor prognosis and limited treatment options.2 Gastric carcinogenesis is a multifactorial and multistep process that involves the activation of oncogenes and inactivation of tumor suppressor genes at different stages of GC progression.3 Identifying key factors involved in the progression of GC and further investigating their molecular mechanisms, not only to predict GC outcome, but may improve diagnosis and explore new therapeutic tools based on these mechanisms.
More recently, obtained from researches suggested that an important role of the endogenous opioid system influencing long‐term outcomes in cancer surgery patients in human cancers.4 The opioid system, consists of three G protein‐coupled receptors, mu, delta, and kappa (MOR, DOR, and KOR, respectively), which are stimulated by a family of endogenous opioid peptides.5 The opioid receptors and their ligands were discovered mostly in the central nervous system and also occur in a wide spectrum of peripheral organs and tissues.6 Several retrospective clinical studies have shown a reduced incidence of cancer recurrence after reduced doses of opioids after the operation.7, 8 In addition, data demonstrate that opioids could induce tumor growth, inhibit apoptosis, and promote angiogenesis.9 That is, opioid receptors expressed in cancer cells may play important roles in cancer progression.
Among opioids system subtypes, the mu‐opioid receptor (MOR), is the main target for opioids such as morphine, fentanyl, and heroin, with nearly two orders of magnitude greater binding affinity compared with the other two opioid receptors (delta and kappa opioid receptors).10 MOR1 (the most abundant MOR transcript that consists of exons 1, 2, 3, and 4) has been found in several types of cancer11, 12, 13 (including breast, colon, lung cancer, and esophageal squamous cell carcinoma) and involved in the cancer progression and metastasis.14 Previous studies showed that the cytoplasmic MOR1 expression may be a high‐risk factor for lymph node metastasis of esophageal squamous cell carcinoma patients.13 More recently, a study performed by Singleton et al. suggested an increased mu‐opioid receptor expression in the progression of metastatic lung cancer.15 Moreover, stimulation of MOR1 in some tumor cells by opioids has been reported to influence tumor cell growth, migration and metastatic spread, indicating that opioids may have direct effects on cancer cell through activation of MOR1.16 Thus, an important role of MOR1 in regulating carcinogenesis and cancer progression has emerged, and MOR1 might become a marker of clinical relevance for cancer patients.
However, to our knowledge, few reports have been published concerning the role of MOR1 in GC, including whether it can represent a prognostic factor. Thus, we explore the expression and functional effects of MOR1 in GC, in order to determine whether opioids will affect GC progression in the subsequent experiment. In our study, we assess MOR1 expression in 154 cases of primary GC using real‐time quantitative PCR (RT‐qPCR) and immunohistochemistry (IHC) assay. The aim was to evaluate the association between MOR1 expression and clinical pathological factors and analyze its impact on survival.
Materials and Methods
Patients
A total of 154 GC patients who underwent surgical resection from February 2002 to January 2007 at Affiliated Hospital of Medical College, Qingdao University were analyzed. Ninety‐three paracancer tissues which were more than 5 cm away from the edge of tumor were randomly selected. Tissue samples were fixed in 10% neutralized formalin and embedded in paraffin for histological processing. None of these subjects had a history of autoimmune or inflammatory diseases. Patients had received preoperative treatment, radiation therapy or chemotherapy were excluded. Data on pathological findings and clinical follow‐up were obtained from medical charts. The stage of GC was classified according to the American Joint Committee on Cancer stage (AJCC, 7th edition). The overall survival (OS) was calculated starting from the date of the initial surgery to the time of death, counting death from any cause as the end point or the last date of follow‐up as the end point, if no event was documented. For patients who remained alive until the cut‐off date, survival data were recorded as 60 months. All patients were followed up until November 2011. The research protocol was approved by the Ethics Committees of the Institute of Oncology and the hospital. Table 1 summarizes the clinical and pathological features of all cases.
Table 1.
Different MOR1 expressions in gastric cancer tissues and paraneoplastic tissues
| MOR1 expression | Gastric cancer | Paraneoplastic tissues | n | χ2 | p |
|---|---|---|---|---|---|
| Negative | 41 | 74 | 115 | 73.775 | <0.001 |
| Weakly positive | 35 | 11 | 46 | ||
| Moderately positive | 52 | 6 | 58 | ||
| Strongly positive | 26 | 2 | 28 | ||
| Total | 154 | 93 | 247 |
Extraction of total RNA and RT‐qPCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Tokyo, Japan), and 5 mg of extracted RNA samples was reverse transcribed into cDNA by a One‐step PrimescriptcDNA Synthesis Kit (TaKaRa, Dalian, China) according to the manufacturer's protocol (Promega, Madison, WI, USA). The resulting cDNAs were subjected to RT‐qPCR analysis to evaluate the relative expression levels of MOR1 and GAPDH (an internal control). Real‐time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Warrington, United Kingdom) on the Stepone plus system (Applied Biosystems) according to the normalized PCR conditions. GAPDH was used for normalization. Primers for MOR1 and GAPDH were from GeneCopoeia (HQP012059, HQP064347). All reactions were run in triplicate.
MOR1 IHC
The expression of MOR1 was detected through IHC analyses with 3‐μm‐thick sections of formalin‐fixed and paraffin‐embedded blocks. For IHC staining, tissue sections were dewaxed in xylene and rehydrated gradually with graded ethanol. For antigen retrieval, all the sections were incubated by microwave oven in citrate buffer solution (pH 6.0) for 20 minutes. Endogenous peroxidase was inactived by 0.3% hydrogen peroxide in methanol for 15 minutes. After that, tissue slides were incubated with rabbit polyclonal antibody against human MOR1 (dilution 1:100, Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C overnight and then visualized antibody binding sites with the SP peroxidase detection system. Finally, sections were incubated in 3,3'‐diaminobenzidine tetrahydrochloride for 3–10 minutes and restained with 0.1% hematoxylin. In every case, negative control reaction was set with PBS replacing MOR1 antibody, while the known positive‐stained section was used as positive control.
Immunohistochemical assessment
Evaluation of MOR1 immunoreactivity was carried out independently by two experienced observers without knowledge of patient evolution. The expression of MOR1 was scored by multiplying the intensity scores and the percentage area positively stained. The immunostaining was read in a semiquantitative manner. The percent positivity was scored as “0” (5%, negative), “1” (5%–25%, sporadic), “2” (25–50%, focal), or “3” (50%, diffuse). The staining intensity was scored as “0” (no staining), “1” (weakly stained), “2” (moderately stained), or “3” (strongly stained). After such calculation, the finally composite scores were divided into four grades: 0–1 were negative (−), 2–4 were weakly positive (+), 5–8 were moderately positive (++), and 9–12 were strongly positive (+++). Two and more than 2 scores were evaluated as positive results. Based on the MOR1 expression levels, the GC patients were divided into two groups: the low MOR1 expression group (MOR1– or MOR1+) and the high MOR1 expression group (MOR1++ or MOR1+++).
Statistical analyses
A paired‐sample t‐test was used to compare the MOR1 mRNA levels in the tumor tissue samples with their adjacent non‐tumorous tissue samples. Pearson's chi‐square test was used for analysis of associations between MOR and clinicopathological characteristics. The OS curves were calculated with the Kaplan–Meier method and were analyzed with the log‐rank test. The Cox proportional hazard analysis was used for univariate and multivariate analyses to explore the effect of the clinicopathological variables and MOR1 expression on survival. All statistical differences were considered significant at the level of p < 0.05.
Results
MOR1 expression in human GC tissues
Tumor cells showed positive diffuse or granular staining for MOR1 that was cytoplasmic and/or membranous (Figure 1). MOR1 was positively stained in the membranous and/or cytoplasm of 73.4% GC cells, whereas the paracancer tissues showed much lower levels of MOR1 with nonspecific weak staining (20.4%; Table 1). Among the 154 tumor samples, 26 cases were detected as strongly positive for MOR1 expression (17%), 52 cases were moderately positive (34%), 35 cases were weakly positive (23%) and 41 cases were negative (27%; Table 1). Overall, the results revealed that the level of MOR1 expression was significantly higher in GC than in paracancer tissues.
Figure 1.
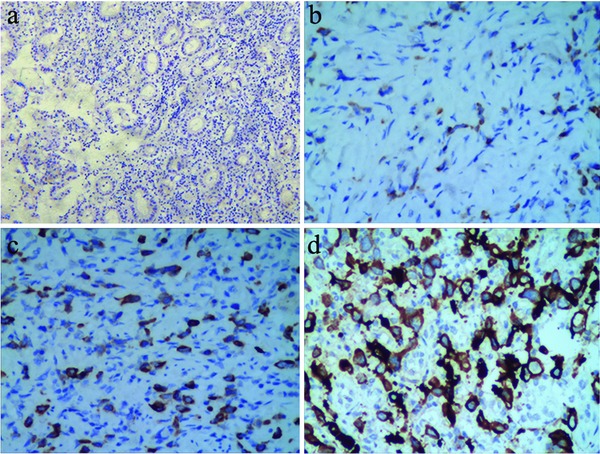
Immunohistochemical staining of MOR1 expression in gastric cancer MOR1 expression was stained in the cytoplasm or membranous of cells. (a) Negative control; (b) Weakly positive staining for MOR1, ×400; (c) Moderately positive staining for MOR1, ×400; (d) Strongly positive staining for MOR1, ×400.
On the other hand, the levels of MOR1 mRNA in 46 pairs of resected specimens (tumor tissue samples and matched adjacent nontumor tissue samples) from eligible GC patients were estimated by RT‐qPCR. As a result, the MOR1 mRNA levels were significantly increased in 30 (65%) of the tumor tissue samples compared with the adjacent nontumor tissue samples (p = 0.03; Figure 2), indicating that MOR1 was commonly overexpressed in human GC.
Figure 2.
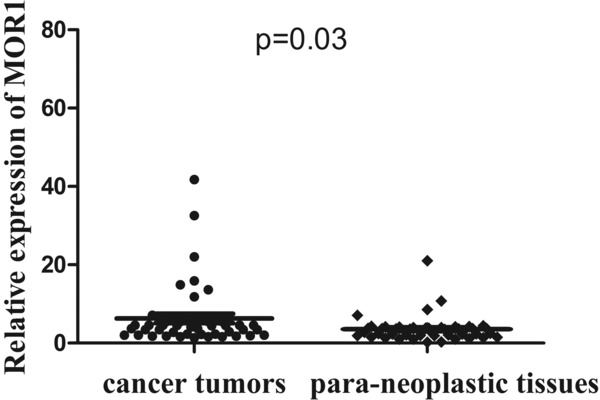
Real‐time quantitative RT‐PCR analysis of MOR1 expression in gastric cancer surgical specimens. The relative mRNA expression of MOR1 was significantly lower in 30 of 46 (65.0%) gastric cancer tissues compared with the corresponding nontumorous tissues. (MOR1/GAPDH; n = 46; p = 0.03)
Correlation between MOR1 expression and clinicopathological characteristics in GC
As our investigation proved that MOR1 expression was increased in GC, which indicated that MOR1 might act as an oncogenic role in GC. We further investigated the association of MOR1 expression with clinicopathological characteristics of patients to explore its potential role in GC progression. Possible associations were analyzed by chi‐square test. Correlations between the MOR1 expression level and clinicopathological characteristics of GC are summarized in Table 2. MOR1 expression was inversely correlated with depth of invasion (p = 0.006), lymph node metastasis (p = 0.001), TNM stage (p = 0.027), and distance metastasis (p = 0.017). However, there was no significant difference in other clinicopathological features such as age (p = 0.468), gender (p = 0.270), tumor size (p = 0.090), tumor differentiation (p = 0.505), venous invasion (p = 0.063), and neural invasion (p = 0.061) between these two groups.
Table 2.
Correlation between MOR1 expression and clinicopathological variables of 154 gastric cancer cases
| Characteristics | No. | MOR1 | p value | |
|---|---|---|---|---|
| Low | High | |||
| All cases | 154 | 41 | 113 | |
| Age (years) | ||||
| <60 | 53 | 16 | 37 | 0.468 |
| ≥60 | 101 | 25 | 76 | |
| Gender | ||||
| Male | 114 | 33 | 81 | 0.270 |
| Female | 40 | 8 | 32 | |
| Tumor size (cm) | ||||
| <5 | 85 | 18 | 67 | 0.090 |
| ≥5 | 69 | 23 | 46 | |
| Tumor differentiation | ||||
| Poor | 47 | 14 | 33 | 0.505 |
| Well/moderate | 107 | 27 | 80 | |
| Depth of invasion | ||||
| T1–T2 | 53 | 7 | 46 | 0.006 a |
| T3–T4 | 101 | 34 | 67 | |
| Lymph node metastasis | ||||
| Negative | 96 | 17 | 79 | 0.001 a |
| Positive | 58 | 24 | 34 | |
| Venous invasion | ||||
| Negative | 94 | 30 | 64 | 0.063 |
| Positive | 60 | 11 | 49 | |
| Neural invasion | ||||
| Negative | 86 | 28 | 58 | 0.061 |
| Positive | 68 | 13 | 55 | |
| Distance metastasis | ||||
| Negative | 136 | 32 | 104 | 0.017 a |
| Positive | 18 | 9 | 9 | |
| TNM stage | ||||
| I–II | 50 | 19 | 31 | 0.027 a |
| III–IV | 104 | 22 | 82 | |
a p < 0.05.
The relationship of MOR1 to OS of patients with GC
Kaplan–Meier analysis was applied to examine the prognostic value of MOR1 expression to OS of patients with GC. Results proved that patients with GC of high MOR1 expression tended to have worse OS: patients with high level of MOR1 expression exhibited significant poorer 5‐year OS compared with patients with low level of MOR1 (33.0% vs. 60.1%; p < 0.001; Figure 3A). As far as clinicopathological characteristics were considered, clinical TNM stage, lymph node status, metastasis were significantly associated with clinical outcome (p < 0.05; Table 3; Figure 3).
Figure 3.
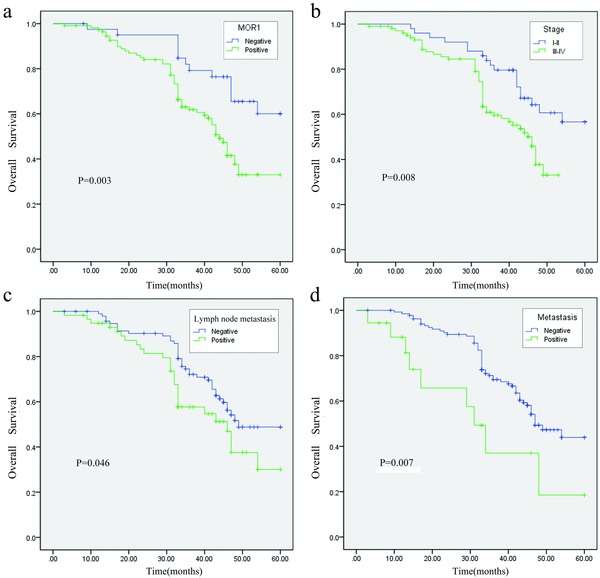
Kaplan–Meier survival curves for postoperational gastric cancer patients. (a) OS curves stratified by MOR1 expression in gastric cancer tissues (low, high expression). (b) Strata based on stage (stage I–II, III–IV); (c) Strata based on lymph node status (negative, positive); (d) Strata based on lymph node status (negative, positive).
Table 3.
Univariate and multivariate analyses of overall survival in patients with gastric cancer
| Variables | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| p value | HR (95% CI) | p value | |
| MOR1 expression (negative/positive) | 0.003 b | 2.529 (1.340–4.769) | 0.004 b |
| Age (<60 years/≥60 years) | 0.916 | ||
| Gender (F/M) | 0.293 | ||
| Tumor size (<5 cm/≥5 cm) | 0.527 | ||
| Tumor differentiation (Poor/well + moderate) | 0.704 | ||
| Depth of invasion (T1–T2/T3–T4) | 0.206 | ||
| Lymph node metastasis (negative/positive) | 0.046 a | 1.552 (0.943–2.556) | 0.084 |
| Venous invasion (negative/positive) | 0.312 | ||
| Neural invasion (negative/positive) | 0.634 | ||
| Distance metastasis (negative/positive) | 0.007 b | 2.736 (1.327–5.644) | 0.006 b |
| TNM stage (I–II/III–IV) | 0.008 b | 1.881 (1.072–3.301) | 0.028 a |
a p < 0.05;
b p < 0.01.
In addition, we performed a multivariate analysis to assess the effect of specific variables on survival, including of TNM stage, lymph node status, metastasis and MOR1 expression, which were found to be significant by univariate analysis. The models showed that MOR1 expression (HR 2.529; 95% confidence interval [CI] 1.340–4.769; p = 0.004) was an independent risk factor predicting GC patients (Table 3). Besides, TNM stage (p = 0.028) and distance metastasis (p = 0.006) were also verified to be independent prognostic factors for the survival in GC patients after multivariate analysis (Table 3; p < 0.05).
Discussion
In current study, we evaluated MOR1 expression in tumors of GC samples to assess whether this information could offer any prognostic value for the management of this cancer type. As a result, we found that the level of MOR1 expression was significantly higher in GC than in paracancer tissues by RT‐qPCR and IHC analysis, respectively. Moreover, an increased expression of MOR1 was significantly associated with deeply tumor invasion, advanced TNM stage, lymph node metastasis and distant metastasis, suggesting that abnormal MOR1 expression might be involved in GC tumor progression and metastasis and MOR1 could also play a tumor suppressor role in GC. Further, we demonstrated that an association between high MOR1 expression and decreased OS in a cohort of 154 GC patients, indicating MOR1 expression was an independent risk factor in GC outcome.
In recent years, it was common knowledge that various clinical parameters related to prognosis, such as tumor size,17 lymph node status,18 which might reflect the frequency of advanced disease at diagnosis and predict tumor recurrence and progression of GC. Thus, identification of some suitable biomarkers for predicting patient prognosis is significant for improving therapeutic effects for patients with advanced GC. MOR1 is a major type of opioid receptors, which was shown to be intergral components of a wide variety of human and animal tumor cells in neural and nonneural tissues.19 It has been found in a wide range of tumors such as lung carcinoma,20 breast cancer,21 prostate cancer,22 liver cancer,23 and colon cancer.24 And previous studies have reported on the role of MOR1 in angiogenesis, tumor cell growth, and metastatic spread.25 However, the clinical significance in gastric carcinogenesis is more complex. In this report, we found significant positive association of MOR1 expression with lymph node metastasis and depth of invasion. Patients with distant metastasis or in the advanced stage also exhibited higher MOR1 expression, suggesting it as a biomarker for tumor metastasis and aggressiveness. In the previous study, Mathew et al. once described MOR is a novel regulator in lung cancer progression.20 In another study carried out by Zhang et al.,13 cytoplasmic MOR1 expression was shown to be a high‐risk factor for lymph node metastasis of the patients. These hypotheses were further provided by our results, common to emphasize the specific involvement of MOR1 in the GC tumor progressive and metastatic procession.
As MOR1 expression was found to be associated with GC invasion and metastasis, it is necessary to considering the crucial factors affecting the prognosis of patients. In order to investigate the prognostic role of MOR1 on GC, we performed Kaplan–Meier analysis of OS. As expected, survival analysis showed that patients with MOR1 positive expression has a significant worse OS compared with those lower expression. In addition, univariate analyses showed that lymph node metastasis, TNM stage, metastasis, and MOR1 expression were significant risk factors affecting OS of GC patients. Importantly, besides TNM stage and metastasis, MOR1 expression was also an independent predictor of OS in gastric adenocarcinoma patients. In light of the evidence discussed here, we suggest that MOR1 may serve as a valuable prognostic biomarker for GC patients after surgery. Previous reports studying the overexpression of MOR induced activation (phosphorylation) of the serine/threonine kinases Akt and mTOR, which are implicated in cancer progression in human nonsmall cell lung cancer.12 Thus, the future research direction is to explore the functional significance of MOR1 overexpression to elucidate a possible mechanism for the findings.
Opiates with clinically relevant concentrations can cause endothelial cell proliferation and migration in vitro, and the selective peripheral opioid receptor (MOR) antagonist, inhibits angiogenesis.26, 27, 28 Among mu agonists, morphine is most relevant in terms of clinical use for cancer pain treatment.29, 30 Morphine essentially activates mu‐receptors in vivo, and the effects of chronic morphine exposure in whole animals reflect the consequences of repeated mu receptor activation in the nervous system.31, 32, 33, 34 Tegeder et al. reported that morphine inhibited tumor cell proliferation at concentrations of >10 μM.35 Intermittent injections of morphine decreased the growth of tumors in a rat model of metastasizing colon cancer.36 On the other hand, Gupta et al.28 demonstrated that morphine, in clinically relevant doses, promoted tumor neovascularization in a human breast tumor xenograft model in mice, leading to increased tumor progression. The discrepancies in results may be due to the differences in administered doses or/and the mode of administration (systemic vs. localized). These examples showed that tumor suppression occurs after chronic high doses of morphine, while tumor‐enhancing effects with morphine occur after a single dose or low daily doses. And, these results were explained, in part, by morphine's potential effects on natural killer cell activity. Our data suggest a possible direct effect of MOR overexpression on GC progression, and further suggest a possible therapeutic role for opioid antagonists that merits further evaluation. Thus, we speculate opioid receptor antagonists such as morphine could be developed for application in a novel GC therapy in the future.
Conclusion
In conclusion, our study indicated that MOR1 was a tumor suppressor and independent prognostic factor for GC patients, which might be a new therapeutic target in GC.
Conflict of Interest
The authors disclose no potential conflicts of interest.
Acknowledgments
This study was supported by National Natural Scientific Foundation of China (NSFC) (No: 81472338); Shandong Excellent Young Scientist Research Award Fund Project (2006BSB14114).
References
- 1. Correa P. Gastric cancer: overview. Gastroenterol Clin North Am 2013; 42: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L, Li JJ, Chen YB, Wang DD, Pan QZ, et al. The prognostic value of tumor‐infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012; 7: e33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CN, Lin JJ, Chen JJ, Lee PH, Yang CY, Kuo ML, Chang KJ, Hsieh FJ. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J Clin Oncol. 2005; 23: 7286–7295. [DOI] [PubMed] [Google Scholar]
- 4. Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011; 106: 814–822. [DOI] [PubMed] [Google Scholar]
- 5. Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol. 1995; 15: 615–635. [DOI] [PubMed] [Google Scholar]
- 6. Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain physician. 2008; 11: S133–S153. [PubMed] [Google Scholar]
- 7. Moss J, Israel RJ. Effects of anesthetics on cancer recurrence. J Clin Oncol. 2009; 27: e89. [DOI] [PubMed] [Google Scholar]
- 8. Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008; 109: 180–187. [DOI] [PubMed] [Google Scholar]
- 9. Gach K, Wyrebska A, Fichna J, Janecka A. The role of morphine in regulation of cancer cell growth. Naunyn Schmiedebergs Arch Pharmacol. 2011; 384: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zadina JE, Kastin AJ, Ge LJ, Hackler L. Mu, delta, and kappa opiate receptor binding of Tyr‐MIF‐1 and of Tyr‐W‐MIF‐1, its active fragments, and two potent analogs. Life Sci. 1994; 55: PL461–466. [DOI] [PubMed] [Google Scholar]
- 11. Fichna J, Janecka A. Opioid peptides in cancer. Cancer Metastasis Rev. 2004; 23: 351–366. [DOI] [PubMed] [Google Scholar]
- 12. Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the mu‐opioid receptor in human non‐small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012; 116: 857–867. [DOI] [PubMed] [Google Scholar]
- 13. Zhang YF, Xu QX, Liao LD, Xu XE, Wu JY, Wu ZY, Shen JH, Li EM, Xu LY. Association of mu‐opioid receptor expression with lymph node metastasis in esophageal squamous cell carcinoma. Dis Esophagus. 2014. Jan 15 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011; 30: 225–238. [DOI] [PubMed] [Google Scholar]
- 15. Singleton PA, Mirzapoiazova T, Hasina R, Salgia R, Moss J. Increased mu‐opioid receptor expression in metastatic lung cancer. Br J Anaesth. 2014; 113(Suppl. 1): i103–i108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lennon FE, Moss J, Singleton PA. The mu‐opioid receptor in cancer progression: is there a direct effect? Anesthesiology. 2012; 116: 940–945. [DOI] [PubMed] [Google Scholar]
- 17. Jiang CG, Xu Y, Wang ZN, Sun Z, Liu FN, Yu M, Xu HM. Clinicopathological analysis and prognostic significance of peritoneal cytology in Chinese patients with advanced gastric cancer. ANZ J Surg. 2011; 81: 608–613. [DOI] [PubMed] [Google Scholar]
- 18. Nesi G, Basili G, Girardi LR, Manetti A, Biliotti G, Barchielli A. Pathological predictors of lymph node involvement in submucosal gastric carcinoma: a retrospective analysis of long‐term outcome. In Vivo. 2009; 23: 337–341. [PubMed] [Google Scholar]
- 19. Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2003. Peptides. 2004; 25: 2205–2256. [DOI] [PubMed] [Google Scholar]
- 20. Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011; 112: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gach K, Piestrzeniewicz M, Fichna J, Stefanska B, Szemraj J, Janecka A. Opioid‐induced regulation of mu‐opioid receptor gene expression in the MCF‐7 breast cancer cell line. Biochem Cell Biol. 2008; 86: 217–226. [DOI] [PubMed] [Google Scholar]
- 22. Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K, Gupta P. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013; 119: 4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu J, Liu Z, Zhao L, Tian H, Liu X, Yuan C. In vivo and in vitro inhibition of human liver cancer progress by downregulation of the mu‐opioid receptor and relevant mechanisms. Oncol Rep. 2013; 30: 1731–1738. [DOI] [PubMed] [Google Scholar]
- 24. Nylund G, Pettersson A, Bengtsson C, Khorram‐Manesh A, Nordgren S, Delbro DS. Functional expression of mu‐opioid receptors in the human colon cancer cell line, HT‐29, and their localization in human colon. Dig Dis Sci. 2008; 53: 461–466. [DOI] [PubMed] [Google Scholar]
- 25. Kuraishi Y. [Effects of morphine on cancer pain and tumor growth and metastasis]. Nihon Rinsho. 2001; 59: 1669–1674. [PubMed] [Google Scholar]
- 26. Singleton PA, Garcia JG, Moss J. Synergistic effects of methylnaltrexone with 5‐fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor‐induced angiogenesis. Mol Cancer Ther. 2008; 7: 1669–1679. [DOI] [PubMed] [Google Scholar]
- 27. Singleton PA, Mambetsariev N, Lennon FE, Mathew B, Siegler JH, Moreno‐Vinasco L, Salgia R, Moss J, Garcia JG. Methylnaltrexone potentiates the anti‐angiogenic effects of mTOR inhibitors. J Angiogene Res. 2010; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival‐promoting signaling and promotes breast tumor growth. Cancer Res. 2002; 62: 4491–4498. [PubMed] [Google Scholar]
- 29. Fallon M, Reale C, Davies A, Lux AE, Kumar K, Stachowiak A, Galvez R, Fentanyl Nasal Spray Study 044 Investigators G. Efficacy and safety of fentanyl pectin nasal spray compared with immediate‐release morphine sulfate tablets in the treatment of breakthrough cancer pain: a multicenter, randomized, controlled, double‐blind, double‐dummy multiple‐crossover study. Journal Support Oncol. 2011; 9: 224–231. [DOI] [PubMed] [Google Scholar]
- 30. Yang Y, Li Y, Huang XE, Lu YY, Wu XY. Exploration of cancer pain treatment by morphine infusion through an embedded device. Asian Pac J Cancer Prev. 2011; 12: 3151–3152. [PubMed] [Google Scholar]
- 31. Fujita W, Gomes I, Devi LA. Mu‐Delta opioid receptor heteromers: New pharmacology and novel therapeutic possibilities. Br J Pharmacol. 2014. Feb 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plante GE, VanItallie TB. Opioids for cancer pain: the challenge of optimizing treatment. Metabolism. 2010; 59( Suppl 1): S47–S52. [DOI] [PubMed] [Google Scholar]
- 33. Haberstock‐Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin‐dependent endocytosis of mu‐opioid receptors in striatal neurons. J Neurosci. 2005; 25: 7847–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carr DJ, Gebhardt BM, Paul D. Alpha adrenergic and mu‐2 opioid receptors are involved in morphine‐induced suppression of splenocyte natural killer activity. J Pharmacol Exp Ther. 1993; 264: 1179–1186. [PubMed] [Google Scholar]
- 35. Tegeder I, Grosch S, Schmidtko A, Haussler A, Schmidt H, Niederberger E, Scholich K, Geisslinger G. G protein‐independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003; 63: 1846–1852. [PubMed] [Google Scholar]
- 36. Yeager MP, Colacchio TA. Effect of morphine on growth of metastatic colon cancer in vivo. Archives of surgery. 1991; 126: 454–456. [DOI] [PubMed] [Google Scholar]


