Abstract
Inflammatory myofibroblastic tumors (IMT) in the head and neck region are rare neoplasms that generally mimic benign/low-grade neoplasms. Overexpression of anaplastic lymphoma kinase (ALK) has been reported in 50% of IMT cases, secondary to ALK activation by structural rearrangements in the ALK gene, which results in a fusion protein with echinoderm microtubule associated protein like 4 (EML4) in ~20% of cases. The present study describes a case of a 74-year-old woman with a malignant IMT in the right posterior hypopharynx harboring a previously unreported chromosomal rearrangement resulting in EML4 and ALK gene fusion. Strong ALK immunoreactivity was observed in neoplastic cells, while fluorescent in situ hybridization combined with fluorescent fragment analysis and direct sequencing identified the first case of the 3a/b variants of the EML4-ALK fusion gene in IMT. The results of the current study highlight the uncommon occurrence of ALK-positive IMT in the head/neck region and demonstrate the importance of integrating different molecular methodologies to identify unequivocal gene fusion characterization.
Keywords: inflammatory myofibroblastic tumor, fusion variants, echinoderm microtubule associated protein like 4, anaplastic lymphoma kinase, molecular markers
Introduction
Inflammatory myofibroblastic tumors (IMT) are distinctive tumor entities described in almost all anatomical sites under several definitions, including inflammatory pseudotumor and plasma cell granuloma. IMT generally shows a benign clinical outcome and is common in children and young adults, although the age of occurrence is extremely broad (1). Presenting symptoms are related to the site of the primary tumor origin, although fever, weight loss, malaise and night sweats are also reported. Surgical resection is the mainstay of treatment, while chemoradiotherapy is generally ineffective (1).
Approximately half of IMT patients carry rearrangements of the anaplastic lymphoma kinase (ALK) gene (2–5). ALK is a tyrosine kinase receptor, which is typically expressed in the central nervous system (3,4). Fusion of the ALK gene with different partners may result in the overexpression of ALK and activation of its kinase domain (6–8). Identification of ALK fusion genes may support a diagnosis of IMT, and additionally allow initiation of an effective treatment regimen with ALK inhibitors (6,7). IMTs occurring in the head and neck region are extremely rare and are more commonly located in the larynx, orbit, paranasal sinus, trachea and parotid gland. The present study describes the characterization of the 3a/b variants of the echinoderm microtubule associated protein like 4 (EML4)-ALK gene fusion, which has not been reported previously in IMT of the hypopharynx.
Materials and methods
Case description
A 74-year-old, non-smoking woman was hospitalized after presenting with weight loss, progressive dysphagia, odynophagia and globus sensation. The patient had an unremarkable medical past and routine laboratory tests, serum tumor markers, a QuantiFERON® tuberculosis test and antineutrophil cytoplasmic antibodies test came back negative. A total-body computed tomography scan revealed a 5-cm mass situated at the right posterior wall of the hypopharynx (Fig. 1A). No other lesions were identified.
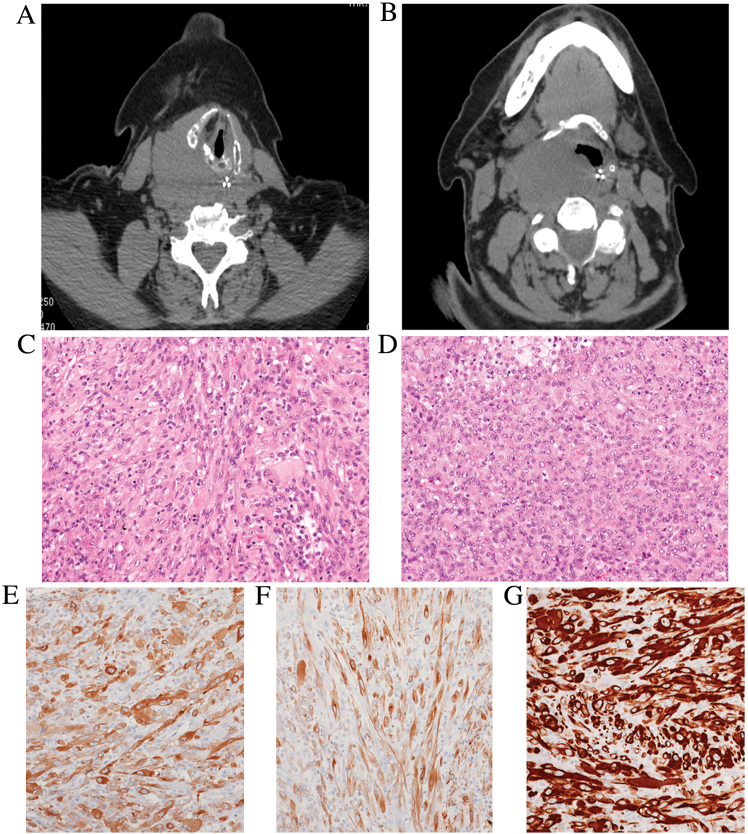
Figure 1.
Computed tomography scans of the (A) head and (B) neck region revealed a poorly-circumscribed mass (maximum diameter, 5 cm), which involved the posterior wall of the right hypopharynx, dislocating the oesophagus and tracheal axis and infiltrating to the soft palate. Hematoxylin and eosin staining of tumor tissue sections revealed that the tumor was composed of (C) spindle-shaped or (D) epithelioid myofibroblasts arranged in fascicular or storiform-like patterns, admixed with scattered lymphocytes, plasma cells and foamy histiocytes (magnification, ×150). Immunohistochemistry demonstrated that the tumor cells expressed (E) smooth muscle actin, (F) desmin and (G) ALK (magnification, ×150). ALK, anaplastic lymphoma kinase.
Radiological examinations demonstrated the impossibility to perform a complete surgical resection since the tumor exhibited infiltrative margins and involved adjacent structures. However, the patient was symptomatic due to the progressive occlusion of the pharynx, which could result in a life-threating condition.
Diagnostic and palliative surgical resection was performed, and the specimen was fixed in 10% neutralized formalin for 24 h at room temperature and embedded in paraffin blocks, which were subsequently cut into 3-µm sections for routine hematoxylin-eosin staining. Examination under a light microscope revealed the proliferation of spindle-to-epithelioid cells intermingled with a mixed inflammatory infiltrate represented by lymphocytes, plasma cells and foamy histiocytes (Fig. 1B-F). Mitotic rate was extremely high (>5 mitoses/high-power field) and focal ulceration of the over lining mucosa was observed. Tumor cells strongly expressed smooth-muscle actin, desmin and ALK (clones ALK1, 5A4 and D5F3), while there was no immunoreactivity for pan-cytokeratins, S100 protein, p63, cluster of differentiation (CD) 21, CD35, CD68 and human herpes virus (HHV)-8. In situ hybridization with an Epstein-Barr virus-encoded small RNA probe came back negative. Subsequently, a diagnosis of IMT with epithelioid features was confirmed. In accordance with the guidelines of the Hospital Institutional Review Board, consent for anonymous research use of the tumor specimen and data collection was obtained. The patient refused enrollment in a clinical trial, which included treatment with ALK inhibitor, and subsequently succumbed to disease progression at 11 months post-diagnosis.
Tissue specimen and cell lines
A representative neoplastic formalin-fixed paraffin-embedded (FFPE) tissue sample from the 74-year-old woman was used as biological material. NCI-H2228 and MRC5 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA) and Sigma-Aldrich (Merck Millipore, Darmstadt, Germany), respectively. The cell lines were cultured under the recommended conditions. Briefly, the NCI-H2228 cell line was cultured in RPMI-1640 medium containing 1% antibiotics (penicillin/streptomycin) and 10% fetal bovine serum (FBS) at 37°C in 5% CO2. The MRC5 cell line was cultured in Eagle's Minimum Essential Medium supplemented with 2 mM glutamine, 1% non essential amino acids and 10% FBS at 37°C in 5% CO2. All media were purchased from Euroclone (EuroClone SpA, Milan, Italy). RNA and cell blocks from the H2228 and MRC5 cell lines were utilized as positive and negative controls for fluorescent in situ hybridization (FISH) analysis and variants 3a/b EML4-ALK fusion.
Immunohistochemistry
Serial 4-µm-thick sections were obtained from FFPE blocks representative of tumor tissue for immunohistochemical analysis. All reactions were performed using a BenchMark XT fully automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA) and antibody incubation was for 8 h at 37°C. The following antibodies were used: Pan-cytokeratins (prediluted; cat. nos. 760–2521 and 790–4555 for clones AE1 and CAM5.2, respectively; Ventana Medical Systems, Inc.), smooth-muscle actin (prediluted; cat. no. 760–2833; Ventana Medical Systems, Inc.), desmin (prediluted; cat. no. 760–2513; Ventana Medical Systems, Inc.), p63 (prediluted; cat. no. 790–4509; Ventana Medical Systems, Inc.), CD21 (prediluted; cat. no. 760–4245; Ventana Medical Systems, Inc.), CD35 (prediluted; cat. no. 760–4439; Ventana Medical Systems, Inc.), HHV-8 (prediluted; cat. no. 760–4260; Ventana Medical Systems, Inc.), ALK (prediluted; cat. nos. 790–2918 and 790–4796 for clones ALK1 and D5F3, respectively; Ventana Medical Systems, Inc.), ALK (1:50 dilution; cat. no. NCL-L-ALK; Novocastra; Leica Microsystems, Inc., Buffalo Grove, IL, USA), S100 (prediluted; cat. no. 760–2523; Ventana Medical System, Inc.) and CD68 (prediluted; cat. no. 790–2931; Ventana Medical Systems, Inc.).
Cytogenetics and ALK break apart FISH assay
FISH assay was performed on 4-µm FFPE sections using Vysis ALK Break Apart FISH Probe kit (Abbott Molecular, Abbott Park, IL, USA). The hybridized slides were reviewed on an Olympus IX-50 microscope (Olympus Corporation, Tokyo, Japan) at ×100 magnification.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA extraction from tumor tissue was performed with the RecoverAll™ Total Nucleic Acid Isolation kit (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. A total of 500 ng RNA was reverse transcribed using the SuperScript® III First-Strand Synthesis system (Invitrogen; Thermo Fisher Scientific, Inc.) for RT-PCR reactions. To analyze variants 1 and 2, two specific synthetic DNA double strands of the EML4-ALK fusion region were synthesized. Amplified products were characterized by cloning PCR products into the StrataClone™ PCR Cloning Vector pSC-A (Agilent Technologies, Inc., Santa Clara, CA, USA), straight amplifying from crude lysates of single bacterial colonies was performed using the following primers: EML4_exon6_F, 5′-CTGCAGACAAGCATAAAGATG-3′ and ALK_exon20_R, 5′-GCTTGCTCAGCTTGTACTC-3′. Direct sequencing was performed using the BigDye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) and an ABI PRISM® 3130 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Fluorescent fragment analysis
Fluorescent PCR amplification was performed using a reverse fluorescein amidite-labeled primer specific to exon 20 of ALK and forward primers specific to exons 13–20 and 6 of EML4 to amplify variants 1, 2 and 3a/b, respectively (8). As a control for RNA quality, primers specific to β-2-microglobulin were employed. RT-PCR products were size-fractionated by capillary electrophoresis in an ABI 3130 Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.) and results were analyzed with GeneMapper® software v.4 (Applied Biosystems; Thermo Fisher Scientific, Inc.).
To confirm the results of fluorescent analysis, DNA fragments were analyzed by microchip electrophoresis using an Agilent 2100 Bioanalyzer with the DNA 1000 assay sizing reagent kit (Agilent Technologies, Inc.).
Results
Detection of ALK involvement in IMT
ALK rearrangement was assessed by FISH analysis. Single and multiple copies of the intact ALK fusion together with the abnormal split pattern were observed in the tumor cells of the tissues specimen with a frequency of 65% ALK rearranged gene (combined EML4-ALK fusion and 5′end-ALK deletion), (Fig. 2A).
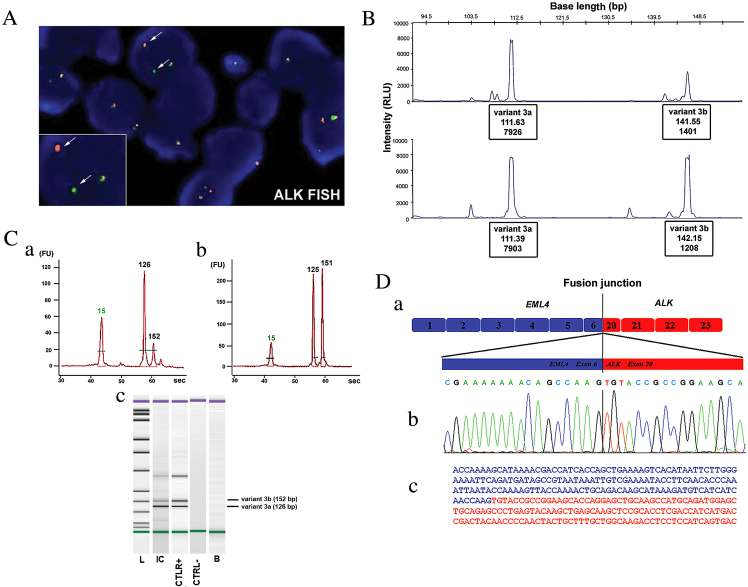
Figure 2.
(A) Molecular cytogenetic analysis of the FFPE tumor sample, in which the disrupted ALK locus was identified by fluorescent in situ hybridization analysis. The white arrows indicate the split of the green and red probe signals (hybridizing to 5′ and 3′ regions of ALK, respectively) in the tumor cells. (B) Fluorescent fragment analysis in the tissue specimen positive for ALK translocation. RNA from the FFPE tissue (upper panel) and NCI-H2228 cell line (lower panel) was amplified by RT-PCR using an unlabeled EML4 exon 6 forward primer and a fluorescein amidite-labeled ALK exon 20 reverse primer. Two peaks were observed (at 112 and 142 bp) indicating the presence of the 3a/b variants of the EML4-ALK fusion. (C) Fluorescent fragment analysis of the (Ca) inflammatory myofibroblastic tumor tissue and (Cb) control NCI-H2228 cell line using the Agilent 2100 BioAnalyzer. Two different fluorescent peaks were observed at 126 and 152 bp, corresponding to the 3a/b variants of the EML4-ALK fusion. (Cc) The peaks were visualized on the gel resulting from the capillary microelectrophoresis. (D) EML4-ALK fusion variant 3a characterization. (Da) Schematic of the EML4-ALK fusion variant 3. (Db) Sequence electropherogram of the RT-PCR product. (Dc) Fusion junction of EML4 exon 6 (blue) and ALK exon 20 (red) sequences. FFPE, formalin-fixed paraffin-embedded; ALK, anaplastic lymphoma kinase; RT-PCR, reverse transcription-polymerase chain reaction.
Identification of the EML4-ALK 3a/b fusion variants
Two fragments of the expected length (112 and 142 bp) corresponding to the fusion 3a/b variants were detected during the first round of RT-PCR, which is designed to detect the most frequent 1,2 and 3a/b types of transcript. Using specific primers for the amplification of the EML4-ALK 3a/b variants fusion cDNA, the same two pair of PCR products were detected in the NCI-H2228 cell line and in the IMT patient sample. These molecular findings were confirmed using two different fluorescent fragment analysis methods; the first was previously described by Sanders et al (8), (Fig. 2B) and the second uses novel, fast and efficient microchip electrophoresis to carefully detect ALK variation through the use of capillary microelectrophoresis and detection (Fig. 2C). Molecular cloning and direct sequencing of each PCR product confirmed the presence of the variant 3a fusion (linking exon 6 of EML4 to exon 20 of ALK) and the 3b fusion, containing an insertion of 33 bp mapped to intron 6 of EML4 that was located between the same exons of EML4 and ALK (Fig. 2D).
Discussion
The World health organization (WHO) classification of soft tissue tumors defines IMT as the neoplastic counterpart among the wastebasket group of lesions previously known as inflammatory pseudotumors (9). IMT represents a proliferation of myofibroblastic cells, often intermingled with lymphocytes, plasma cells and histiocytes, which eventually forms a highly aggressive lesion (9). IMT is ubiquitous and margin-free surgical resection is considered the only curative treatment. By contrast, aggressive or metastatic lesions have no standard therapy and chemotherapy is usually ineffective.
In the head and neck region, the larynx is the most common location of IMT, while a few cases in the hypopharynx have been reported (10,11). Clinical and radiological differential diagnosis is broad and includes infections (tuberculosis and mycoses), Wegener's granulomatosis, amyloidosis and other malignancies, such as carcinoma, melanoma and salivary gland tumors (10,11).
Up to 70% of IMTs harbour ALK gene rearrangements resulting in the formation of a chimeric fusion protein, which is detectable by FISH or immunohistochemistry (2–5). ALK is a receptor-type protein tyrosine kinase that is currently being analysed by oncologists as an essential growth driver, which defines its potential susceptibility to ALK inhibitors (7,12–15). The oncogenic function of ALK occurs as a result of it forming a fusion protein with various gene partners through chromosomal translocations, most commonly TPM3 or TPM4 in IMT, resulting in oncogenic activation of ALK (3). Alternatively, a small inversion [inv2(2)(p21p23)] on the short arm of chromosome 2 leads to a functional EML4-ALK fusion-type tyrosine kinase (3–22) (Table I). Initially described in non-small cell lung carcinoma (15), this abnormality was also identified in ~20% of ALK-rearranged pulmonary IMT cases (5). To date, a total of 10 cases of EML4-ALK fusions in IMT have been reported (3,5,9). The present study described a EML4-ALK rearranged IMT located in the hypopharynx. By using two different fluorescent fragmentation analysis methods, the specific 3a/b variants were identified within the RNA of the tumor specimen and confirmed by standard Sanger sequencing. This description of the ALK fusion 3a/b variants in an IMT case, together with the few cases of ALK-EML4 fusions reported in literature, corroborates the existing hypothesis that identical ALK fusions detected in different tumor types may drive an inappropriate activation of the same kinase signalling pathway, which could be oncogenic in disparate cellular lineages. In addition, the present case also noted the epithelioid appearance of the tumor cells, which could potentially lead to a dismal clinical course. Notably, Mariño-Enríquez et al (23) described ALK-positive intra-abdominal IMT composed predominantly of sheets of round-to-epithelioid cells significantly associated with aggressive course with rapid local recurrences.
Table I.
ALK fusion partners in IMT.
| Authors, year | IMT location | Patient age/gender | ALK fusion | Refs. |
|---|---|---|---|---|
| Lawrence et al, 2000 | Abdomen | 23 years/F | TMP3-ALK | (3) |
| Lung | 30 years/F | TMP3_ALK | (3) | |
| Abdomen | 1 year/M | TMP4-ALK | (3) | |
| Bridge et al, 2001 | Neck | 3 years/F | CLTC-ALK | (16) |
| Pelvis | 37 years/M | CLTC-ALK | (16) | |
| Cools et al, 2002 | Abdomen | 4 months/M | CARS-ALK | (17) |
| Ma et al, 2003 | Abdomen | 1 year/M | RANBP2-ALK | (18) |
| Abdomen | 7 months/M | RANBP2-ALK | (18) | |
| Debiec-Rychter et al, 2003 | Bladder | 46 years/M | ATIC-ALK | (19) |
| Panagopoulos et al, 2006 | Abdomen | 23 years/M | SEC31L1-ALK | (20) |
| Takeuchi et al, 2011 | Lung | 45 years/M | PPFIBP1-ALK | (21) |
| Lung | 34 years/F | PPFIBP1-ALK | (21) | |
| Wang et al, 2012 | Neck | 7 years/F | DCTN1-ALK | (22) |
| Lovly et al, 2014 | Lung | 38 years/F | EML4-ALK | (12) |
| Mesentery | 11 years/F | LMNA-ALK | (12) | |
| Shoulder | 1 year/F | PRAKAR1A-ALK | (12) | |
| Bladder | 26 years/F | FN1-ALK | (12) | |
| Pelvis | 14 years/M | TFG-ALK | (12) |
ALK, anaplastic lymphoma kinase; IMT, inflammatory myofibroblastic tumors; F, female; M, male.
In conclusion, to the best of our knowledge, the present study describes for the first time the presence of the EML4-ALK 3a/b variant in a case of malignant IMT of the hypopharynx. This was achieved by integrating different methodologies, which is considered the most suitable molecular approach for gene fusion characterization. Furthermore, the identification of ALK rearrangement in cases of malignant IMTs may offer a rationale to adopt selective targeted therapies in this ‘orphan’ tumor.
Acknowledgements
The present study was supported by the Italian Ministry of Health (grant nos. RC1503LO51, 2010-2316264 and RC1502AP18), the ‘5×1000′ voluntary contributions and Associazione Italiana per la Ricerca sul Cancro (grant no. 12983).
References
- 1.Surabhi VR, Chua S, Patel RP, Takahashi N, Lalwani N, Prasad SR. Inflammatory myofibroblastic tumors: Current update. Radiol Clin North Am. 2016;54:553–563. doi: 10.1016/j.rcl.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 3.Lawrence B, PerezAtayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CD, Fletcher JA. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffin CM, Patel A, Perkins S, ElenitobaJohnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 5.Antonescu CR, Suurmeijer AJ, Zhang L, Sung YS, Jungbluth AA, Travis WD, AlAhmadie H, Fletcher CD, Alaggio R. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957–967. doi: 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mano H. ALKoma: A cancer subtype with a shared target. Cancer Discov. 2012;2:495–502. doi: 10.1158/2159-8290.CD-12-0009. [DOI] [PubMed] [Google Scholar]
- 8.Sanders H, Li H, Bruey JM, Scheerle JA, MeloniEhrig AM, Kelly JC, Novick C, Albitar M. Exon scanning by reverse transcriptase polymerase chain reaction for detection of known and novel EML4-ALK fusion variants in non-small cell lung cancer. Cancer Genet. 2011;204:45–52. doi: 10.1016/j.cancergencyto.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher C, Bridge JA, Hogendoorn PC, et al. WHO Classification of Tumours of Soft Tissue and Bone. IARC; Lyon: 2013. [Google Scholar]
- 10.Graefe H, Stellmacher F, Sotlar K, Wollenberg B, Gehrking E. Inflammatory pseudotumor of the hypopharynx: Clinical diagnosis, immunohistochemical findings and treatment of this rare disease. In Vivo. 2008;22:817–820. [PubMed] [Google Scholar]
- 11.Nakayama K, Inoue Y, Aiba T, Kono K, Wakasa K, Yamada R. Unusual CT and MR findings of inflammatory pseudotumor in the parapharyngeal space: Case report. AJNR Am J Neuroradiol. 2001;22:1394–1397. [PMC free article] [PubMed] [Google Scholar]
- 12.Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller VA, Coffin CM. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889–895. doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, Hill DA. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: A comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ni C, Xu YY, Zhou SH, Wang SQ. Differential diagnosis of inflammatory myofibroblastic tumour and low-grade myofibroblastic sarcoma: Two case reports with a literature review. J Intern Med Res. 2011;39:311–320. doi: 10.1177/147323001103900134. [DOI] [PubMed] [Google Scholar]
- 15.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 16.Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. 2001;159:411–415. doi: 10.1016/S0002-9440(10)61711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, De Wolf-Peeters C, Pauwels P, Hagemeijer A, Marynen P. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2002;34:354–362. doi: 10.1002/gcc.10033. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, Zuppan C, Bridge JA. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–1053. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 19.Debiec-Rychter M, Marynen P, Hagemeijer A, Pauwels P. ALK-ATIC fusion in urinary bladder inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;38:187–190. doi: 10.1002/gcc.10267. [DOI] [PubMed] [Google Scholar]
- 20.Panagopoulos I, Nilsson T, Domanski HA, Isaksson M, Lindblom P, Mertens F, Mandahl N. Fusion of the SEC31L1 and ALK genes in an inflammatory myofibroblastic tumor. Int J Cancer. 2006;118:1181–1161. doi: 10.1002/ijc.21490. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi K, Soda M, Togashi Y, Sugawara E, Hatano S, Asaka R, Okumura S, Nakagawa K, Mano H, Ishikawa Y. Pulmonary inflammatory myofibroblastic tumor expressing a novel fusion, PPFIBP1-ALK: Reappraisal of anti-ALK immunohistochemistry as a tool for novel ALK fusion identification. Clin Cancer Res. 2011;17:3341–3381. doi: 10.1158/1078-0432.CCR-11-0063. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Krishnan C, Nguyen EP, Meyer KJ, Oliveira JL, Yang P, Yi ES, EricksonJohnson MR, Yaszemski MJ, Maran A, Oliveira AM. Fusion of dynactin 1 to anaplastic lymphoma kinase in inflammatory myofibroblastic tumor. Hum Pathol. 2012;43:2047–2052. doi: 10.1016/j.humpath.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Mariño-Enríquez A, Wang WL, Roy A, LopezTerrada D, Lazar AJ, Fletcher CD, Coffin CM, Hornick JL. Inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135–144. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]