Abstract
Background:
Chronic obstructive pulmonary disease (COPD) attribute to systemic inflammation which is responsible for microalbuminuria reflecting endothelial dysfunction, could be a significant surrogate marker of potential cardiovascular morbidity.
Objective:
The aim of our study was to find out the possible association of COPD with early cardiovascular changes in the form of renal endothelial dysfunction.
Settings and Design:
Case–control, multi-group, cross-sectional hospital-based study was designed and conducted in the Department of Respiratory Medicine of BPS Government Medical College for Women, Khanpur Kalan, Sonipat, Haryana.
Subjects and Methods:
The study included 150 subjects, comprising of three groups with each having 50 subjects: Group 1 – acute exacerbation of COPD, Group 2 – stable COPD patients, Group 3 – asymptomatic smokers. Pulmonary function test, urine albumin creatinine ratio (UACR) and brachio-ankle pulse wave velocity were measured in all the subjects.
Statistical Analysis:
Data were analyzed using SPSS ver 20 (IBM, USA) software. Continuous variables were compared by unpaired Student's t-test while correlation was measured by Pearson correlation test, P < 0.05 was considered statistically significant.
Results:
The mean urine albumin creatinine ratio UACR value in acute exacerbation of COPD (283.30 mg/g; standard deviation [SD] ±871.98) was found significantly higher compare to control subjects (24.17 mg/g; SD ± 32.105;) P = 0.038. Besides this COPD patients with Type 2 respiratory failure having robust positive correlation in between UACR and arterial blood pH (r = 0.559; P = 0.030) while it was inverse and moderate with partial pressure of arterial oxygen (r = −0.470; P = 0.077).
Conclusions:
Acute state of COPD with or without Type 2 respiratory failure is having a significant impact on cardiovascular system in the form of early microvascular changes.
KEY WORDS: Endothelium, inflammation, lung, microvascular, obstructive diseases
INTRODUCTION
As per the WHO, chronic obstructive pulmonary disease (COPD) will become the third leading cause of death by 2030.[1] COPD is characterized by inhaled particles and gasses inducing chronic inflammation of the airways accompanied by an airflow limitation that is not fully reversible.[2,3] Although COPD is associated with an abnormal inflammatory response in the lungs, it demonstrates important extrapulmonary manifestations and multiple comorbidities for which several cytokines and inflammatory mediators have been implicated.[4,5] The link between COPD and its systemic expressions is still not well understood.[6,7]
Systemic inflammation has an important role in the pathogenesis of COPD. Circulating proinflammatory cytokines and C-reactive protein (CRP), a derivative of these cytokines, are important markers of systemic inflammation.[8,9] Cardiovascular disease being the major cause of mortality in COPD, particularly in patients with mild to moderate COPD.[10,11,12]
It was cited in recent research that microalbuminuria (MAB) was increased in exacerbation periods of COPD.[13,14] While the link between lower forced expiratory volume in 1 s (FEV1) and emphysema severity with endothelial dysfunction also endorsed by researchers.[15,16] Furthermore, MAB was also considered to be related to endothelial dysfunction.[17] It was studied that MAB is related with increased glomerular filtration and consequent protein leakage due to increased hypoxemia during COPD episodes.[13,14]
Similarly in few studies, it was endorsed that MAB has a stronger association with cardiovascular events and death than CRP, therefore, it is an emerging therapeutic target for primary prevention strategies.[18,19,20,21] Hence, we aim to investigate the presence and degree of proteinuria by estimating urine albumin creatinine ratio (UACR) in a group of patients with chronic obstructive lung diseases with or without respiratory failure and its possible association with central arterial stiffness with the help of noninvasive multiparameter comprehensive cardiovascular analysis.
SUBJECTS AND METHODS
The study was conducted in the Department of Respiratory Medicine, BPS Government Medical College for Women, Khanpur Kalan, Sonipat, Haryana.
A hospital-based multi-group case–control type, cross-sectional study was designed which included the patients visiting the outpatient department (OPD) as well as admitted to the ward and respiratory Intensive Care Unit. The study subjects were comprised into three groups, each group having fifty subjects:
Acute exacerbation of COPD (AECOPD)
Stable COPD (SCOPD) patients visiting OPD in follow-up
Asymptomatic smoker.
Study subjects
Diabetic patient, pregnant female, patients who do not fit the criteria to perform pulmonary function test (PFT), HIV patients, patient with renal failure, history of ischemic heart diseases, body mass index >30 kg/m2 and <15 kg/m2, patient with urinary tract infection, age <35 years, not given written consent were excluded from the study.
Written informed consent of all subjects, as well as Institutional Ethics Committee approval, was taken before starting the project.
All subjects will be evaluated by taking a detailed clinical history. Complete routine as well as specific investigations were done such as MAB, UACR, electrocardiogram, lipid, profile, PFT, arterial blood gas (ABG) analysis, blood sugar, kidney function test, brachio-ankle pulse wave velocity (baPWV), serum electrolytes (Na+, K+, Ca2+), and skiagram of chest in posterioranterior view.
For the diagnosis of MAB, care was taken when collecting samples for the urine UACR. An early morning sample was preferred. The patient was instructed to avoid heavy exercises 24 h before the test. The UACR is inaccurate in a person with too much or too little muscle mass due to the variation in creatinine level, which is produced by the muscle.
MAB was defined as UACR ≥3.5 mg/mmol (female) or ≥2.5 mg/mmol (male), or, with both substances measured by mass, as a UACR between 30 and 300 µg albumin/mg creatinine.[22] An alternative definition of MAB is a UACR on a random urine sample of more than 30 mg (but <300 mg) of albumin/g of creatinine.[23]
Cardiovascular assessment
The baPWV were measured using a volume plethysmographic apparatus Periscope (Recorders and Medicare System Pvt. Ltd., Chandigarh, India). baPWV was used as an index of arterial stiffness. To measure baPWV, cuffs were applied to both brachia and ankles, and all blood pressures were measured simultaneously by cuff-oscillometric method. The pulse volume waveforms were also recorded simultaneously using a plethysmographic sensor connected to the cuffs. baPWV was calculated from the time interval between the wave fronts of the brachial and ankle waveforms, and the path length from the brachia to ankle (0·597 × height + 14·4014).[24]
Pulmonary function
Pulmonary function was measured by a forced vital capacity (FVC) maneuver on a computed spirometer with automated quality checks (BTL-08 Spiro PC, manufactured by Health and Medical Industry, United Kingdom, Airflow limitation was defined as a ratio of FEV1 to FVC of <70%. The severity of airflow limitation was graded by the ratio of FEV1 to FEV1 predicted value as per the global initiative for chronic obstructive lung disease:[25]
Stage 1: FEV1 ≥80% of predicted-mild
Stage 2: FEV1 50≤FEV1 <80% of predicted-moderate
Stage 3: FEV1 30≤FEV1 <50% of predicted severe
Stage 4: FEV1 <30% of predicted-very severe.
Pulmonary function was measured by trained medical technologist according to a standardized protocol.
Statistical analysis
The analysis was done using standard statistical software (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: USA). All data were expressed as mean ± standard deviation. Unpaired Student's t-test was used to compare means and Pearson correlation analysis was performed to determine whether the PFT indices, baPWV, UACR results correlated with the ABG parameters, P < 0·05 was considered to be statistically significant.
RESULTS
A total of 150 study subjects, who visited to the OPD as well as admitted to the Department of Respiratory Medicine during January 2015 to November 2015 were included and comprises in three groups of 50 subjects as per study design (rewrite). All study groups were matched for age and sex and other sociodemographic profile to alleviate the effect of confounding factors. The mean age in SCOPD patients was 60.2 ± 10.36 while it was 62.22 ± 12.02 among subjects of AECOPD and 60.1 ± 9.17 in the control group. Male: female ratio and smoking index were also matched [Table 1]. Mean fasting blood sugar for AECOPD and SCOPD group was 104.06 ± 22.42 mg/dl and 96.38 ± 14.91 mg/dl, while mean cholesterol and triglyceride for AECOPD group was 164.84 ± 23.09 mg/dl and 135.8 ± 20.70 mg/dl, which was similar to SCOPD group (cholesterol 160.2 ± 22.63; triglyceride 132.26 ± 18.58) [Table 1]. Mean value of serum electrolyte (Na+, K+, Ca2+) among AECOPD group was 133.378 ± 18.011 mmol/L, 3.51 ± 0.622 mmol/L; 0.945 ± 0.187 mmol/L.
Table 1.
Characteristics of the 150 subjects presented as mean±standard deviation with P value of significance
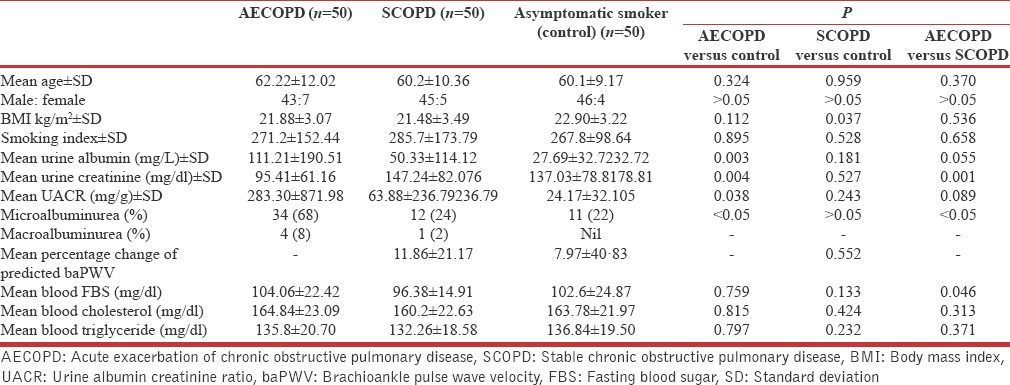
The mean UACR among SCOPD subjects (63.88 ± 236.79 mg/g) was higher than control asymptomatic smokers (24.17 ± 32.10 mg/g) though it was found statistically insignificant (t = 1.175, P = 0.243). This figure was quite high among the subjects of AECOPD 283.30 ± 871.98 mg/g and it was significantly high compared to control group (t = 2.10, P = 0.038), although it was statistically insignificant in comparison to SCOPD subjects (t = 1.71, P = 0.089) [Figure 1]. Besides this in SCOPD group, the MAB was found only in 12 (24%) subjects, near to control group 11 (22%), but it was quite high among AECOPD subjects 34 (68%) P < 0.05 and only 1 (2%) subject having macroalbuminuria in SCOPDstable COPD group, while it was 4 (8%) in AECOPD group, however, it was nil among asymptomatic smokers.
Figure 1.
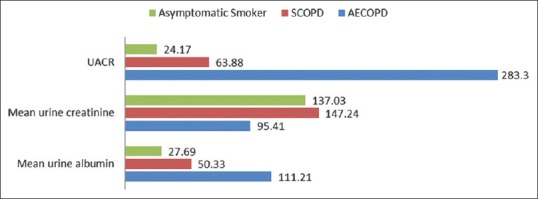
Graphical presentation of urine albumin creatinine ratio level and its component among various study groups
The mean percentage change of predicted in baPWV, a robust predictor of arterial stiffness was found high among SCOPD subjects (11.86 ± 21.17) in comparison to asymptomatic smokers (7.97 ± 40.83) although it was found statistically insignificant (P = 0.552).
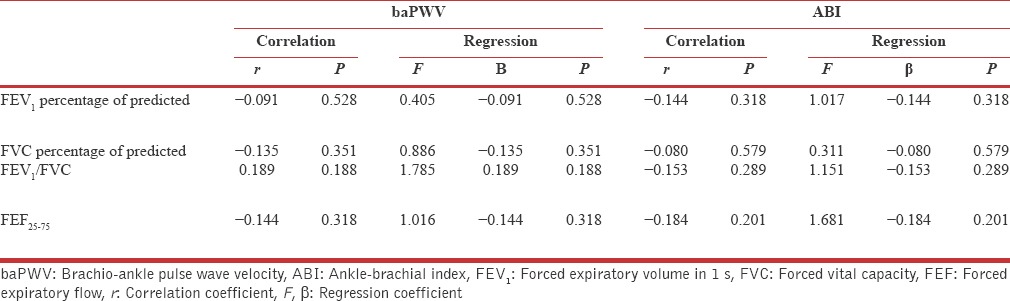
Although no correlation found in between UACR and baPWV among SCOPD subjects (r = 000, P = 0.999), however very weak negative correlation was found in between ankle-brachial index and UACR (r = −0.034, P = 0.815) besides this no significant correlation was measured in between PFT indices and UACR among SCOPD subjects [Table 2].
Table 2.
Regression and correlation analysis for urine albumin creatinine ratio with physiological variables in subjects with stable chronic obstructive pulmonary disease (n=50)
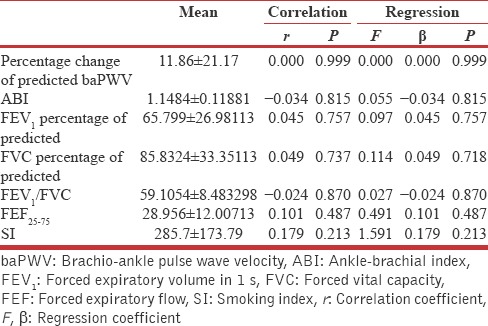
It was also found that there is no significant dependency of UACR on baPWV and PFT indices by analyzing regression coefficient [Table 3]. In AECOPD group, UACR was significantly affected by the pH of blood and had a robust and significant positive correlation (r = 0.288, P = 0.043) [Figure 2] besides this, it was found to be a dependent variable on pH of blood at statistically significant level, while it shows an insignificant correlation as well as regression with partial pressure of arterial oxygen (PaO2) and partial pressure of arterial carbon dioxide (PaCO2) [Table 3].
Table 3.
Regression and correlation analysis for urine albumin creatinine ratio with arterial blood gas indices in subjects with acute exacerbation of chronic obstructive pulmonary disease (n=50)
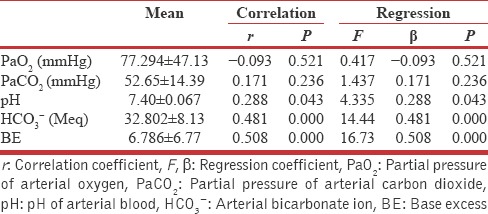
Figure 2.
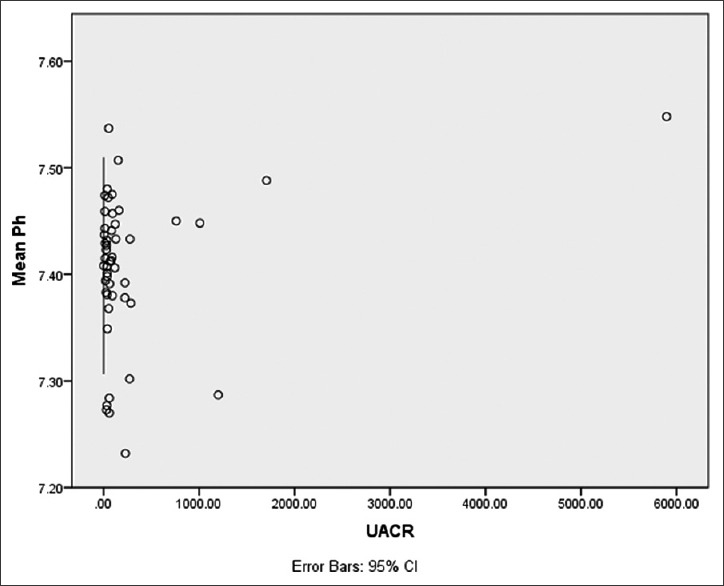
Scattered plot showing correlation of urine albumin creatinine ratio with blood pH in acute exacerbation of chronic obstructive pulmonary disease
In AECOPD group, 15 (30%) subjects were found in critical state of Type 2 respiratory failure according to ABG report (mean pO2: 51.13 ± 7.51 mmHg; mean partial pressure carbon dioxide: 59.95 ± 8.96 mmHg, mean pH: 7.409 ± 0.07) and they were put on noninvasive ventilator at bi-level mode, out of them 13 (86.66%) subjects were detected positive for MAB and 2 (13.33%) were having macroalbumin urea.
The mean UACR was also quite high 595.42 ± 1525.17 mg/g among COPD subjects who were diagnosed as Type 2 respiratory failure. There was moderate negative correlation of UACR with PaO2 (r = −0.470, P = 0.077) and positive correlation with PaCO2 (r = 0.331, P = 0.228) among Type 2 respiratory failure cases. However, the mean score of UACR was quite high for Type 2 respiratory failure cases compare to non-Type 2 respiratory failure, though it was statistically insignificant (595.42 ± 1525.17 mg/g, 149.53 ± 276.75; t = 1.688; P = 0.098), while correlation of UACR with ABG indices was found statistically significant only with Type 2 respiratory failure subjects [Table 4]. It was also recorded that there is very weak negative correlation in between diminution of airflow and baPWV [Table 5].
Table 4.
Regression and correlation analysis for urine albumin creatinine ratio with arterial blood gas indices in subjects with Type 2 respiratory failure and non-Type 2 respiratory failure among acute exacerbation of chronic obstructive pulmonary disease
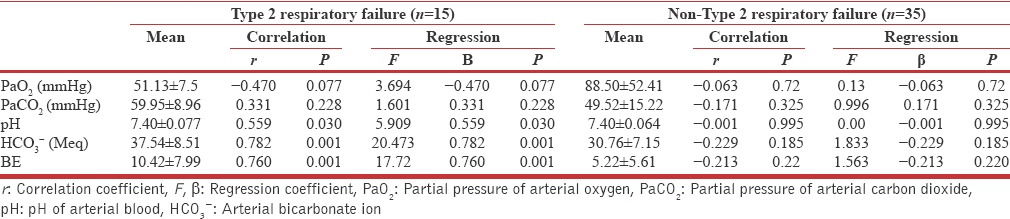
Table 5.
Regression and correlation analysis for Brachio-ankle pulse wave velocity and ankle-brachial index with pulmonary function test indices in subjects with stable chronic obstructive pulmonary disease (n=50)
DISCUSSION
It is an emerging phenomenon that COPD has its systemic effects in the form of structural and biochemical alterations in the organs other than lungs.[6] It has been cited that the vascular endothelium is a major site in which the systemic effects of inflammation occur, and hence MAB is an indirect manifestation of the effect of systemic inflammation on renal endothelial permeability and renal perfusion.[17] In this study, the level of UACR was found much higher in COPD with acute exacerbation group compare to both SCOPD as well as asymptomatic smokers or control group, though that elevation was statistically significant only with controls. It was also observed that the subjects of AECOPD with Type 2 respiratory failure have quite higher UACR value compare to non-Type 2 respiratory failure cases among AECOPD group (specify). Similar results observed by Cogo et al.[13] in his retrospective study on 177 subjects where they also found significant correlation with urinary protein and PaO2 level, however, contrary to the present study, it was similar for both groups, normoxemic (PaO2 >60 mmHg) as well as hypoxemic (PaO2 <60 mmHg).
UACR was found sensitive to change in blood pH and was significantly correlated with it, besides this by the regression analysis it was also observed that UACR is significantly dependent variable on the change in blood pH and blood bicarbonate ion level, although this correlation was more pronounced in Type 2 respiratory failure cases than non-Type 2 cases. Polatli et al.[14] suggest that ABG value may affect renal function due to increased sympathetic activity and hypoxemia which causes increased capillary permeability resulting in proteinuria. However, in this study, blood carbon dioxide and blood oxygen level was moderately correlated with UACR in subjects with Type 2 respiratory failure while it was found very weak in between UACR and blood gases in non-Type 2 respiratory failure subjects, furthermore, it was also observed that UACR was significantly sensitive to change in pH of blood and bicarbonate. Recently, a study conducted by Bulcun et al. found an inverse correlation of UACR with PaO2.[26] However, some patients with hypoxemia did not show increased albuminuria probably due to other factors such as genetic susceptibility to oxidative stress may play an important role.[27]
Inverse relation of PaO2 with UACR in subjects with Type 2 respiratory failure may indicate UACR as an effective predictor of the risk of developing multiple organ dysfunction syndrome and determine the responsiveness of the therapy of the patients with Type 2 respiratory failure in an early stage.
Although baPWV was cited as good predictor of central arterial stiffness by McAllister et al.,[28] and many other researchers, however in present study baPWV was inversely correlated with diminution of airflow, besides this there was no significant association recorded between the UACR and central arterial stiffness [Table 2].
While in similar studies conducted by John et al. and Refaat et al. found that MAB is independently related to aortic stiffness in patients with COPD, however the percentage change of predicted value of pulse wave velocity was not calculated by them for the final analysis of correlation, which may be consider as more specific data not affected by ethnic variables.[29,30]
By the knowledge of previous research, it is evident that tobacco consumption decreases the glomerular filtration rate, and renal blood flow, which further increases renovascular resistance and thereafter cause the thickening of renal arteriole, lead to hypoperfusion of parenchyma, the resulting repeated hypoperfusion may cause glomerular damage which increases albumin excretion.[31] Since this study smoking shows very weak positive correlation with urinary excretion of albumin among SCOPD group, though it was found statistically insignificant.
In a large Multi-Ethnic Study of Atherosclerosis prospective cohort study conducted by Harris et al.[32] among 6814 subjects found that UACR was significantly associated with FEV1 (P = 0.002) and FEV1/FVC ratio (P = 0.04), while in present study only weak inverse correlation was observed with FEV1/FVC ratio and insignificant association was recorded in between diminution of airflow in all stages of COPD with urinary excretion of albumin, which reveal that airflow obstruction does not affect the microvascular changes significantly until it may lead to severe hypoxia or hypercarbia in the state of acute exacerbation or respiratory failure.
CONCLUSIONS
Our study highlights that cardiovascular changes start at microvascular level in the early stage of COPD, however, they become more significant and pronounced in AECOPD with type 2 respiratory failure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–21. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 2.Vijayan VK. Chronic obstructive pulmonary disease. Indian J Med Res. 2013;137:251–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Asche CV, Leader S, Plauschinat C, Raparla S, Yan M, Ye X, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2012;7:201–9. doi: 10.2147/COPD.S25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–12. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 5.Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22:809–14. doi: 10.1183/09031936.03.00031403. [DOI] [PubMed] [Google Scholar]
- 6.Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 7.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist AS, Calverley P, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for chronic obstructive pulmonary disease (GOLD). Workshop summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [Update 2009S] [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–85. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 9.Laratta CR, van Eeden S. Acute exacerbation of chronic obstructive pulmonary disease: Cardiovascular links. Biomed Res Int 2014. 2014:528789. doi: 10.1155/2014/528789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of comorbidities. Eur Respir J. 2006;28:1245–57. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 12.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 13.Cogo A, Ciaccia A, Legorini C, Grimaldi A, Milani G. Proteinuria in COPD patients with and without respiratory failure. Chest. 2003;123:652–3. doi: 10.1378/chest.123.2.652. [DOI] [PubMed] [Google Scholar]
- 14.Polatli M, Cakir A, Cildag O, Bolaman AZ, Yenisey C, Yenicerioglu Y. Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis. 2008;26:97–102. doi: 10.1007/s11239-007-0073-1. [DOI] [PubMed] [Google Scholar]
- 15.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: The Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–7. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension. 2005;45:198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 17.Pedrinelli R, Dell’Omo G, Penno G, Mariani M. Non-diabetic microalbuminuria, endothelial dysfunction and cardiovascular disease. Vasc Med. 2001;6:257–64. doi: 10.1177/1358836X0100600410. [DOI] [PubMed] [Google Scholar]
- 18.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 19.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 20.Casanova C, Celli BR. Microalbuminuria as a potential novel cardiovascular biomarker in patients with COPD. Eur Respir J. 2014;43:951–3. doi: 10.1183/09031936.00015614. [DOI] [PubMed] [Google Scholar]
- 21.Romundstad S, Naustdal T, Romundstad PR, Sorger H, Langhammer A. COPD and microalbuminuria: A 12-year follow-up study. Eur Respir J. 2014;43:1042–50. doi: 10.1183/09031936.00160213. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 23.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–10. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 24.Tabara Y, Muro S, Takahashi Y, Setoh K, Kawaguchi T, Terao C, et al. Airflow limitation in smokers is associated with arterial stiffness: The Nagahama study. Atherosclerosis. 2014;232:59–64. doi: 10.1016/j.atherosclerosis.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Revised. 2011. [Last accessed on 2013 Nov 27]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html .
- 26.Bulcun E, Ekici M, Ekici A, Kisa U. Microalbuminuria in chronic obstructive pulmonary disease. COPD. 2013;10:186–92. doi: 10.3109/15412555.2012.735292. [DOI] [PubMed] [Google Scholar]
- 27.Casanova C, de Torres JP, Navarro J, Aguirre-Jaíme A, Toledo P, Cordoba E, et al. Microalbuminuria and hypoxemia in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:1004–10. doi: 10.1164/rccm.201003-0360OC. [DOI] [PubMed] [Google Scholar]
- 28.McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:1208–14. doi: 10.1164/rccm.200707-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John M, Hussain S, Prayle A, Simms R, Cockcroft JR, Bolton CE. Target renal damage: The microvascular associations of increased aortic stiffness in patients with COPD. Respir Res. 2013;14:31. doi: 10.1186/1465-9921-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Refaat A, Abdou M, Ismae A, Alhelali I. Aortic stiffness and microalbuminuria in patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2015;64:541–9. [Google Scholar]
- 31.Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, et al. Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol. 2011;6:2462–9. doi: 10.2215/CJN.00700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris B, Klein R, Jerosch-Herold M, Hoffman EA, Ahmed FS, Jacobs DR, Jr, et al. The association of systemic microvascular changes with lung function and lung density: A cross-sectional study. PLoS One. 2012;7:e50224. doi: 10.1371/journal.pone.0050224. [DOI] [PMC free article] [PubMed] [Google Scholar]


