Abstract
Objective
To detect the relationship between molecular subtypes of breast cancer with expressions of androgen receptor, cytokeratin 5/6 (CK5/6)and Ki-67.
Materials and Methods
Expressions of androgen receptor, CK-5/6 and Ki-67 were determined by immunohistochemistry in paraffin-embedded sections obtained from 86 invasive breast cancer cases of stages I/IIa/IIb in 4 molecular subtypes. Patients treated for recurrent disease and locally advanced disease were excluded.
Results
Forty one luminal A cases, ie. positive estrogen receptor(ER) and/or progesteron receptor (PR) with negative epidermal growth factor receptor (HER2), 14 luminal B, ie. positive ER and/or PR and positive HER2, 14 HER2-enriched (HER2+), ie. negative ER and PR with positive HER2, and 17 triple negative (negative ER and PR and HER2) invasive breast cancers were included. Mean follow-up was 17.46±11.70 mo. Androgen receptor-negativity and CK5/6-positivity were significantly more common in HER2+ and triple negative groups. Ki-67 and histological grade were higher in HER2+ group, significantly. Two deaths were triple negative (P=0.04). Androgen receptor-negativity, CK5/6 and Ki-67 status did not affect survival or systemic metastases, significantly. All groups had local recurrences. Local recurrence was significantly associated with androgen receptor-negativity in luminal A and high Ki-67 value in HER2+ groups. Systemic metastases were significantly more common in triple negative and HER2+ groups.
Conclusion
Molecular subtypes of breast cancer are prognostic and predictive. Androgen receptor is expressed more commonly in luminal subtypes with better prognosis and androgen receptor negativity is associated with development of local recurrence in luminal A cancers.
Keywords: Androgen receptor, cytokeratin, ki-67 antigen, molecular typing, breast cancer
Introduction
Current parameters determining prognosis and adjuvant therapy protocol in breast cancer are patient’s age, tumor size, histological grade, lymph node metastasis, status of estrogen receptor (ER) and/or progestereone receptor (PR) and of human epidermal growth factor receptor-2 (HER2) (1). Molecularly distinct subtypes of breast cancer have been described by gene expression studies: hormone receptor-positive “luminal A”(LA) and “luminal B” (LB) cancers, hormone receptor-negative but HER2 overexpressing and/or amplified “HER2-enriched” cancers (HER2+), and triple negative cancers (TN) negative for both hormone receptors and HER2.
Common immunohistochemical surrogates used by many investigators to discriminate those subtypes are ER, PR, HER2, Ki-67, cytokeratins (CK5/6) and epidermal growth factor receptor (EGFR) (2). HER2 is negative in LA cancers and positive in LB, while both luminal subtypes express ER and/or PR. However, in St Galen Consensus 2013 some modifications were made in description of luminal cancers which is shown in Table 1 (3). HER2+ and TN cancers are hormone receptor-negative. TN tumors are known as basal-like when cytokeratins (CK5/6, CK17, CK14) or EGFR is expressed and as normal-like, when not (4–5).
Table 1.
Characteristics of the molecular subtypes (St Galen Consensus 2013)
| Molecular Subtypes | Characteristics |
|---|---|
| Luminal A |
Luminal A-like: Estrogen and Progesterone receptor positive HER2 negative Ki-67 “low” “Low” recurrence risk on multi-gene expression analysis (if available) |
| Luminal B |
Luminal B-like (HER2 positive): Estrogen receptor positive HER2 over-expressed or amplified Any Ki-67 value, any progesterone receptor status Luminal B-like (HER2 negative): Estrogen receptor positive HER2 negative At least one of: “High” Ki-67 value Progesterone receptor negative or low “High” recurrence risk on multigene analysis (if available) |
| HER2 overexpression |
HER2 positive (non-luminal): Estrogen and Progesterone receptor negative HER2 over-expressed or amplified |
| Basal-like |
“Triple negative (ductal)”: Estrogen receptor negative Progesterone receptor negative HER2 negative |
Overall and recurrence-free survivals are better in LA subgroup (6) while hormone-negative HER tumors and basal-like tumors of TN group have poor prognosis (7).
Androgens influence growth of normal and malignant breast cells. They inhibit basal and estradiole-stimulated proliferation by an androgen receptor (AR)-dependent mechanism (8) and also stimulate growth of breast cancer cells by aromatization to estrogens (9). AR is expressed in 70–90% of invasive breast cancers, while frequency of expression of ER is 60–80% and of PR, 50–70% (10). AR expression is usually associated with low tumor grade, small size, improved response to hormone therapy in hormone responsive cases and longer patient survival (9–11). It has been reported that approximately 1/3 of ER-negative, high grade invasive ductal cancers and 75% of breast cancer metastases expressed AR (10, 11). In TN cancers, AR expression has been reported with different incidences ranging from 13% to 45% (10–13).
In this study we aimed to detect the clinical significance of AR, CK5/6 and Ki-67 by correlating them with clinicopathological parameters in molecular subtypes of breast cancer.
Materials and Methods
Eighty-six invasive breast cancer cases operated between January 2007 and December 2010 were retrospectively evaluated. Patients treated for recurrent disease and those who received neoadjuvant therapy for locally advanced disease were excluded. Initial patient data were available from patient files as well as histopathological features of the disease. As the number of cases was low, molecular subtypes were determined as luminal A (ER and/or PR− positive and HER2− negative), luminal B (ER and/or PR− positive and HER2− positive), HER2 overexpressive (ER− negative, PR− negative and HER2− negative), and triple negative (ER− negative, PR− negative and HER2− negative). Patients without followup data were excluded. Mean follow-up period was 16.49±12.61 mo in LA, 15.14±8.25 mo in LB, 18.21±16.14 mo in HER2+ and 20±9.82 mo in TN group (P=0.676).
Expressions of AR, Ki-67, CK5/6, ER, PR and HER2 were detected in formalin-fixed, paraffin-embedded sections of surgically resected breast cancer specimens using immunohistochemistry. Primary antibodies were used for Ki-67 (Thermo SP6: Thermo-Fischer Scientific, Fremont, United States of America), for CK5/6 (Thermo D516B4: Thermo-Fischer Scientific, Fremont, USA), for ER (Thermo SP1: Thermo-Fischer Scientific, Fremont, USA), for PR (Thermo SP2: Thermo-Fischer Scientific, Fremont, USA), for HER2 (Thermo neu AB-17: Thermo-Fischer Scientific, Fremont, USA) and for AR (Novocastra NCC-AR-318: Leica Biosystems Ltd, Newcastle, UK). Immunohistochemistry was performed on 4μm-thick paraffin sections as the standard protocol according to individual data-sheets of each antibody by using Ventana Benchmark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA). However, immunostaining for AR was applied manually because autostaining failed. For this purpose after treatment with 3% hydrogen peroxide solution, sections were pretreated for antigen retrieval in citrate buffer at pH 6.0 in the microwave oven. Primary antibodies were incubated overnight at 40ºC and then sections were treated with diaminobenzidine as chromogen. Sections were counterstained with hematoxylin.
Cases were labeled as positive for AR, ER and PR when 1% or more tumor cells expressed the marker (Figure 1 and Figure 2). HER2 immunohistochemical staining was scored from 0 to +3 according to the guideline indicated for HercepTestTM (DAKO) (14). It was considered positive when strong membranous staining (3+) was observed and negative, when the score was 0 or 1+. Fluorescence in situ hibridization test was used for those with 2+ staining. Ki-67 expression was quantified by visual grading. An estimated percentage of Ki-67-positive cells was determined and scored according to increasing percentage intervals as low (0 to 14%) or high (15 and more) Ki-67 expression (15) (Figure 3 and Figure 4). Any staining for CK5/6 was accepted as positive (Figure 5 and Figure 6).
Figure 1.
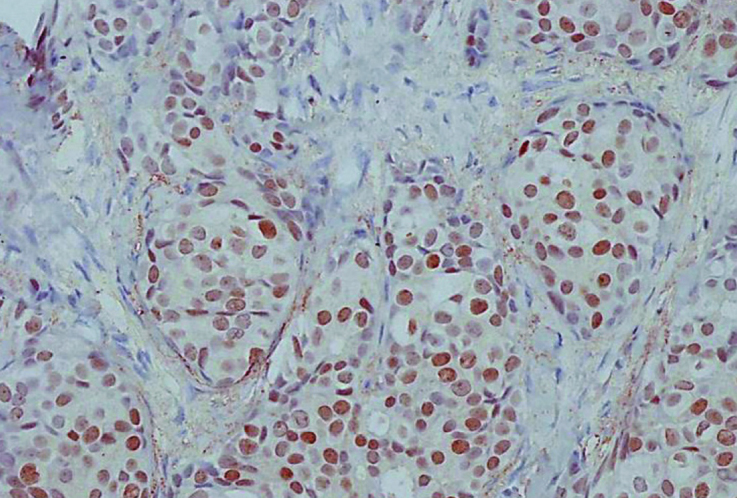
Expression of androgen receptor by 90% in luminal tumor (IHC, 200×)
Figure 2.

Expression of CK 5/6 in triple negative tumor (IHC, 400×)
Figure 3.
High Ki-67 level (75%) in luminal tumor (IHC, 400×)
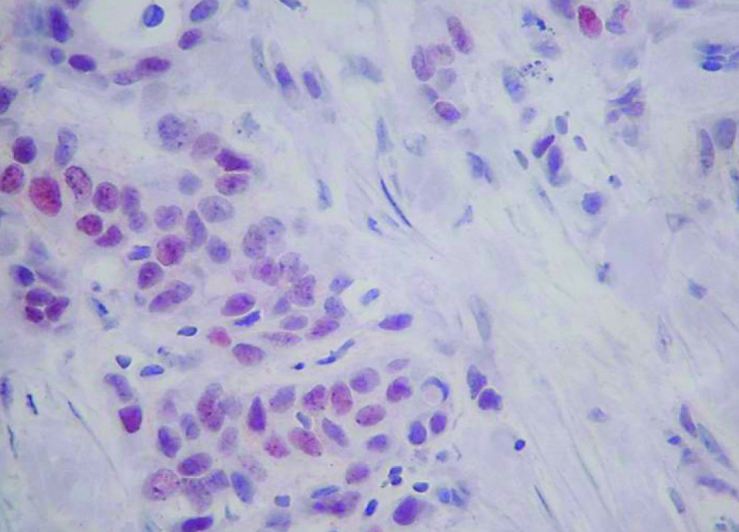
Figure 4.
Low Ki-67 value (10%) in triple negative tumor (IHC, 400×)
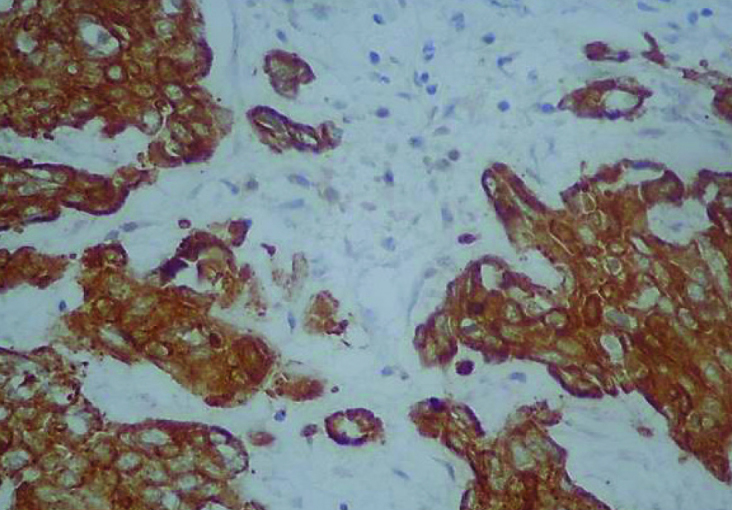
Figure 5.
Expression of CK 5/6 in HER2 overexpressing tumor (IHC, 400×)
Figure 6.
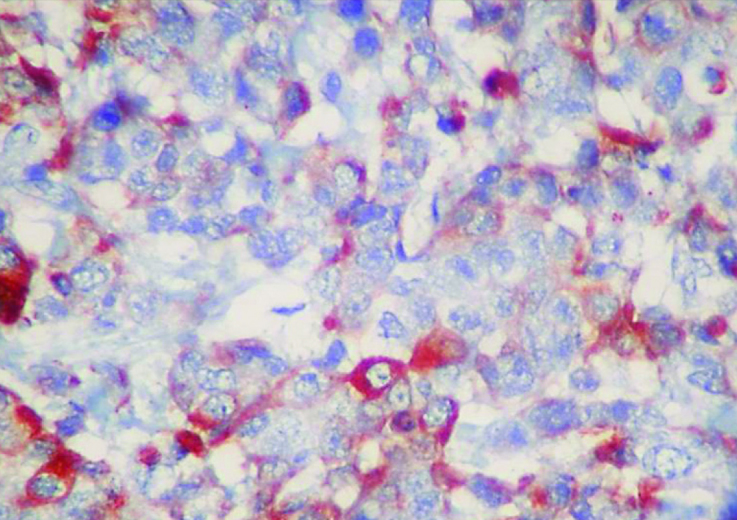
Loss of expression of androgen receptor in triple negative tumor (IHC, 200×)
Disease free and overall survivals were determined. Clinicopathological factors were compared between 4 subgroups. Then effects of AR, CK5/6 and Ki-67 expression on survival and on development of local and systemic relapse were determined.
The study was financially supported by Bezmialem Vakif University, Unit of Scientific Research Project (BAP) with the project number of 6.2011/23.
Statistical Analysis
Statistical calculations were performed with (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA) program for Windows. Standard descriptive statistical calculations (mean, standard deviation) were used, the variables indicated a normal distribution. One way ANOVA test was used in the comparison of the groups, the variables did not indicate a normal distribution. Kruskal Wallis test was used in comparison of the groups and Chi-square and Fischer’s exact test was used for evaluation of the qualitative data. Statistical significance level was established at P<0.05.
Results
Eighty-six invasive breast cancer cases of stages I, IIA and IIB were included. In all cases lumpectomy with clear margins was the operation performed. Axillary dissection was applied in 46 cases either as the primary procedure or after detection of metastasis in sentinel lymph node. All had radiotherapy but chemotherapy and hormone therapy were performed according to disease characteristics.
Molecular subtypes were LA in 41 cases (47.7%), LB in 14 (16.27%), HER2+ in 14 (16.27%) and TN in 17 (19.76%).
No significant difference was observed in the mean age of the patients and mean tumor size (F=1.47, P=0.229 and F=0.68, P=0.567, respectively). No systemic metastasis was observed in LB. Mean disease free survival was 15.83±12.36 mo in LA, 14.86±7.67 mo in LB, 15±12.4 mo in HER2+ and 17.06± 9.77 mo in TN cases (F=0.13, P=0.944). Results of the one way analysis of variance is demonstrated in Table 2.
Table 2.
One way analysis of variance of several parameters of 4 molecular subgroups
| LA | LB | HER2+ | TN | P | |
|---|---|---|---|---|---|
| Age (yr) | 54.22±13.59 | 49.07±13.55 | 49.29±9.29 | 56.88±12.79 | P=0.229 |
| Tumor size (mm) | 24.49±11.92 | 26.71±12.98 | 23.14±12.06 | 29.13±16.65 | P=0.567 |
| Time to local relapse (mo) | 16.15±12.62 | 14.86±7.67 | 16.29±12.33 | 19.71±9.63 | P=0.644 |
| Time to systemic relapse (mo) | 15.83±12.36 | 14.86±7.67 | 15±12.4 | 17.18±9.9 | P=0.935 |
| Disease free survival (mo) | 15.83±12.36 | 14.86±7.67 | 15±12.4 | 17.06±9.77 | P=0.944 |
LA: Luminal A; LB: Luminal B; HER2+: HER2-enriched; TN: Triple negative
AR positivity was significantly more common in LA and LB groups than in HER2+ and TN (P=0.0001). CK5/6 was positive more commonly in HER2+ and TN groups (P=0.001). Higher Ki-67 value (in 74% of the cases) and high histological grade were detected more commonly in HER2+ group (P=0.04, P=0.034, respectively). Tumor type and rates of lymphovascular invasion and multifocal tumor were similar between groups. TN group had 2 deaths (P=0.04). Rate of local recurrence was not different between groups (P=0.383). Metastases were signifantly more common in HER2+ and TN groups (P=0.002). Comparision of the clinicopathological parameters together with AR, CK5/6 and Ki-67 status between 4 groups were demonstrated in Table 3.
Table 3.
Comparison of the molecular subgroups for several parameters
| LA (N:41) | LB (N:14) | HER2+ (N:14) | TN (N:17) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| Characteristics | N | % | N | % | N | % | N | % | P | |
| Androgen Receptor | (−) | 4 | 9.80 | 8 | 57.10 | 10 | 71.40 | 14 | 82.40 | P=0.0001 |
| (+) | 37 | 90.20 | 6 | 42.90 | 4 | 28.60 | 3 | 17.60 | ||
| CK 5/6 | (−) | 40 | 97.60 | 13 | 92.90 | 9 | 64.30 | 11 | 64.70 | P=0.001 |
| (+) | 1 | 2.40 | 1 | 7.10 | 5 | 35.70 | 6 | 35.30 | ||
| Ki-67 | Low | 28 | 68.30 | 6 | 42.90 | 4 | 28.60 | 11 | 64.70 | P=0.04 |
| High | 13 | 31.70 | 8 | 57.10 | 10 | 71.40 | 6 | 35.30 | ||
| Multifocal Tumor | (−) | 28 | 68.30 | 9 | 64.30 | 8 | 57.10 | 16 | 94.10 | P=0.104 |
| (+) | 13 | 31.70 | 5 | 35.70 | 6 | 42.90 | 1 | 5.90 | ||
| Tumor Type | Ductal | 33 | 80.50 | 13 | 92.90 | 14 | 100 | 14 | 82.40 | P=0.170 |
| Lobular | 3 | 7.30 | 0 | 0 | 0 | 0 | 3 | 17.60 | ||
| Other | 5 | 12.20 | 1 | 7.10 | 0 | 0 | 0 | 0 | ||
| Histologic Grade | Low | 32 | 78.00 | 9 | 64.30 | 5 | 35.70 | 10 | 58.80 | P=0.034 |
| High | 9 | 22.00 | 5 | 35.70 | 9 | 64.30 | 7 | 41.20 | ||
| Lymphovascular Invasion | (−) | 28 | 68.30 | 4 | 28.60 | 8 | 57.10 | 8 | 47.10 | P=0.062 |
| (+) | 13 | 31.70 | 10 | 71.40 | 6 | 42.90 | 9 | 52.90 | ||
| In Situ Component | (−) | 9 | 22.00 | 1 | 7.10 | 2 | 14.30 | 12 | 70.60 | P=0.0001 |
| (+) | 32 | 78.00 | 13 | 92.90 | 12 | 85.70 | 5 | 29.40 | ||
| Number of (+) Nodes | (−) | 18 | 43.90 | 3 | 21.40 | 10 | 71.40 | 9 | 52.90 | P=0.139 |
| 1–4 | 13 | 31.70 | 5 | 35.70 | 2 | 14.30 | 2 | 11.80 | ||
| >4 | 10 | 24.4 | 6 | 42.90 | 2 | 14.30 | 6 | 35.30 | ||
| Local Relapse | (−) | 40 | 97.60 | 13 | 92.90 | 12 | 85.70 | 15 | 88.20 | P=0.383 |
| (+) | 1 | 2.40 | 1 | 7.10 | 2 | 14.30 | 2 | 11.80 | ||
| Systemic Metastasis | (−) | 39 | 95.10 | 14 | 100 | 13 | 92.90 | 11 | 64.70 | P=0.002 |
| (+) | 2 | 4.90 | 0 | 0 | 1 | 7.10 | 6 | 35.30 | ||
| Survival Status | Dead | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 11.80 | P=0.04 |
| Alive | 41 | 100 | 14 | 100 | 14 | 100 | 15 | 88.20 | ||
LA: Luminal A; LB: Luminal B; HER2+: HER2-enriched; TN: Triple negative
Two deaths were from TN group. Both were AR negative but survival in TN group was not significantly related to AR-negativity (χ2:0.49, P=0.486). CK5/6 was positive in one death and Ki-67 value was low in both. CK5/6-positivity and Ki-67 value had no association with survival in TN group (χ2:0.21, P=0.643 and χ2:1.24, P=0.266, respectively) (Table 4).
Table 4.
Relation of survival with androgen receptor status, CK 5/6 positivity and Ki67 value
| LA | LB | HER2+ | TN | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| dead | alive | dead | alive | dead | alive | dead | alive | P | ||
| AR | − | 0 | 4 | 0 | 8 | 0 | 10 | 2 | 12 | P=0.486 |
| + | 0 | 37 | 0 | 6 | 0 | 4 | 0 | 3 | ||
| CK 5/6 | − | 0 | 40 | 0 | 13 | 0 | 9 | 1 | 10 | P=0,643 |
| + | 0 | 1 | 0 | 1 | 0 | 5 | 1 | 5 | ||
| Ki-67 | low | 0 | 28 | 0 | 6 | 0 | 4 | 2 | 9 | P=0.266 |
| high | 0 | 13 | 0 | 8 | 0 | 10 | 0 | 6 | ||
AR: Androgen receptor; LA: Luminal A; LB: Luminal B; HER2+: HER2-enriched; TN: Triple negative
AR was negative in all 6 patients with local recurrence. Only in LA group, association of AR negativity with development of local recurrence was statistically significant. Seven of the 9 cases with systemic metastasis were AR negative, which was not significant (Table 5). CK 5/6 positivity was not significantly associated with development of local recurrence or systemic metastasis (Table 6). Both cases with local recurrence in HER2+ group had high Ki-67 value, which was significant. There was no significant relation between Ki-67 value and development of systemic metastasis in groups (Table 7).
Table 5.
Effect of androgen receptor expression on development of local recurrence and systemic metastasis
| Relapse (+) | No relapse | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Subtype | AR Status | N | % | N | % | P | |
| Local Relapse | LA | (−) | 1 | 100 | 3 | 7.50 | P=0.002 |
| (+) | 0 | 0 | 37 | 92.50 | |||
| LB | (−) | 1 | 100 | 7 | 53.80 | P=0.369 | |
| (+) | 0 | 0 | 6 | 46.20 | |||
| HER2+ | (−) | 2 | 100 | 8 | 66.70 | P=0.334 | |
| (+) | 0 | 0 | 4 | 33.30 | |||
| TN | (−) | 2 | 100 | 12 | 80.00 | P=0.486 | |
| (+) | 0 | 0 | 3 | 20.00 | |||
| Systemic Relapse | LA | (−) | 0 | 0 | 4 | 10.30 | P=0.634 |
| (+) | 2 | 100 | 35 | 89.70 | |||
| LB | (−) | - | 0 | 8 | 57.10 | ||
| (+) | - | 0 | 6 | 42.90 | |||
| HER2+ | (−) | 1 | 100 | 9 | 69.20 | P=0.512 | |
| (+) | 0 | 0 | 4 | 30.80 | |||
| TN | (−) | 6 | 100 | 8 | 72.70 | P=0.159 | |
| (+) | 0 | 0 | 3 | 27.30 | |||
AR: Androgen receptor; LA: Luminal A; LB: Luminal B; HER2+: HER2-enriched; TN: Triple negative
Table 6.
Effect of CK5/6 expression on development of local recurrence and systemic metastasis
| Relapse (+) | No relapse | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Subtype | CK5/6 Status | N | % | N | % | P | |
| Local Relapse | LA | (−) | 1 | 100 | 39 | 97.50 | P=0.873 |
| (+) | 0 | 0 | 1 | 2.50 | |||
| LB | (−) | 1 | 100 | 12 | 92.30 | P=0.773 | |
| (+) | 0 | 0 | 1 | 7.70 | |||
| HER2+ | (−) | 1 | 50 | 8 | 66.70 | P=0.649 | |
| (+) | 1 | 50 | 4 | 33.30 | |||
| TN | (−) | 1 | 50 | 10 | 66.70 | P=0.643 | |
| (+) | 1 | 50 | 5 | 33.30 | |||
| Systemic Relapse | LA | (−) | 2 | 100 | 38 | 97.40 | P=0.819 |
| (+) | 0 | 0 | 1 | 2.60 | |||
| LB | (−) | - | 0 | 13 | 92.90 | ||
| (+) | - | 0 | 1 | 7.10 | |||
| HER2+ | (−) | 1 | 100 | 8 | 61.50 | P=0.439 | |
| (+) | 0 | 0 | 5 | 38.50 | |||
| TN | (−) | 3 | 50 | 8 | 72.70 | P=0.349 | |
| (+) | 3 | 50 | 3 | 27.30 | |||
LA: Luminal A; LB: Luminal B; HER2+: HER2-enriched; TN: Triple negative
Table 7.
Effect of Ki-67 value on development of local recurrence and systemic metastasis
| Relapse (+) | No relapse | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Subtype | Ki-67 Status | N | % | N | % | P | |
| Local Relapse | LA | Low | 0 | 0 | 28 | 70.00 | P=0.137 |
| High | 1 | 100 | 12 | 30.00 | |||
| LB | Low | 0 | 0 | 6 | 46.20 | P=0.369 | |
| High | 1 | 100 | 7 | 53.80 | |||
| HER2+ | Low | 0 | 0 | 10 | 83.30 | P=0.016 | |
| High | 2 | 100 | 2 | 16.70 | |||
| TN | Low | 1 | 50 | 10 | 66.70 | P=0.643 | |
| High | 1 | 50 | 5 | 33.30 | |||
| Systemic Relapse | LA | Low | 2 | 100 | 26 | 66.70 | P=0.323 |
| High | 0 | 0 | 13 | 33.30 | |||
| LB | Low | - | 0 | 6 | 42.90 | ||
| High | - | 0 | 8 | 57.10 | |||
| HER2+ | Low | 0 | 0 | 4 | 30.80 | P=0.512 | |
| High | 1 | 100 | 9 | 69.20 | |||
| TN | Low | 5 | 83.30 | 6 | 54.50 | P=0.235 | |
| High | 1 | 16.70 | 5 | 45.50 | |||
LA: Luminal A; LB: Luminal B; HER2+: HER2-enriched; TN: Triple negative
Discussion and Conclusion
Clinical behavior of breast cancer changes with its molecular subtype. Analysis of 12 studies evaluating data from a total of 10,105 breast cancer cases revealed that 78% of the total was luminal tumors and 92% of the luminal cases were LA (16). In different studies approximately 15% of the cases were HER2+ and another 15%, TN (17). Special importance has been given to HER2+ and TN subgroups as both have poor prognosis and TN cancers have no specific targeted therapy (1).
In our study, molecular subtypes were LA in 47.7%, LB in 16.27%, HER2+ in 16.27% and TN in 19.76%. HER2+ group had high histological grade and high Ki-67 value more commonly than other subgroups. Also other characteristics demonstrating poor prognosis were observed significantly more in HER2+ and TN subgroups, such as, CK5/6 expression, AR negativity and development of systemic metastases, in correlation with other previous studies. Also both deaths were TN.
Mortality in subgroups of breast cancer was reported to be time-dependent (16). Within the first years after diagnosis of cancer, it was reported that both HER2+ and TN subgroups had more mortality than LA tumors. It was also demonstrated that LB tumors had poorer prognosis than both HER2+ and LA tumors, and that within TN group basal-like tumors had worse prognosis than non-basal tumors. However, the differences in mortality rates between subgroups reversed after 5–10 years of diagnosis (15, 18). Effects of ER, PR, HER2 and CK5/6 on prognosis were also reported to change with time (7, 16, 19). Thus research on prognostic differences among molecular subtypes requires large groups with prolonged follow-ups.
Short follow-up period in our study limited the ability to compare survival between the subgroups. The only 2 deaths observed were from TN subgroup. Deaths were recorded at the 24th and 28th months of initial diagnosis supporting the previous studies emphasizing the risk within the first years in HER2+ and TN subgroups.
The biomarker used in discrimination of basal tumors, CK5/6, is associated with aggressive behavior and increased risk for systemic metastases (20, 21). Basal-like tumors formed approximately 58% of the TN subgroup and had poorer prognosis. However, basal markers did not affect the prognosis significantly within HER2+ subgroup (16). In our study as the number of cases was low, we did not have the chance to divide TN subgroup as basal and non-basal tumors. CK5/6 expression was more common in HER2+ and TN subgroups, but development of local recurrence or systemic metastasis was not significantly affected by its expression which might be due to short follow-up period.
Ki-67 is a cell proliferation marker in breast cancer and also a biomarker used in identification of LB tumors (1). It has been reported that high Ki-67 expression is significantly associated with breast cancer recurrence and death (22, 23). After neoadjuvant endocrine therapy, changes in Ki-67 expression may help predict long-term outcome (24). In our study expression of high Ki-67 value was observed significantly more commonly in HER2+ subgroup (71%). Both cases with local recurrence in HER subgroup had significantly high Ki-67 expression, but development of systemic metastases was not found to be related to high Ki-67 expression in either subgroup.
AR expression is common in well differentiated breast cancers which are positive for ER and PR (10, 13, 24). Loss of AR expression is usually associated with early onset, high grade tumors negative for ER, PR and HER2 (12). Although the lowest AR expression was observed in TN cancers (24), up to 30% of them were positive for AR (10, 13, 25). That’s why it was suggested that AR could be a potential target in management of AR-positive TN breast cancers in future. In our study AR was more commonly expressed in LA cancers (90%) and rate of expression in TN subgroup was 18%.
Several studies suggested that AR expression can have prognostic role, especially in ER-positive cancers (12, 26). It was reported that higher expression of AR was associated with lower rate of local recurrence and death (27). Thus, AR expression in hormone responsive cases has recently been suggested as an independent prognostic factor for better disease free survival like ER status (28). In our study, AR was also positive in 78% of all hormone responsive cases. Observation of AR negativity was noteworthy in all 6 patients with local recurrence, 7 of the 9 cases with systemic metastasis and both dead cases. In LA subgroup, AR negativity in presence of ER/PR-positivity was significantly related to development of local recurrence. Low number of patients with short follow-up period in the study might be the reason for the insignificant results obtained.
The fact that AR is also detected in 9% to 56% of ER/PR-negative breast cancers made AR expression important (5, 10, 25, 29). AR-positivity was reported to be a significant prognostic factor for disease free survival also in cases negative for ER (11). Many investigators suggested AR as a potential therapeutic target in AR-positive, ER-negative cancers (30). ER-negative cases, when AR is also negative, have an aggressive course with less clinical response to hormonal therapy (31). In our study AR expression was detected in 20% of the cases negative for both ER and PR. Four of the 6 local recurrences, 7 of the 9 systemic metastases and both of the deaths were also negative for ER and PR.
Our study supported previous studies that molecular subtypes of breast cancer are prognostic and predictive. AR was expressed more commonly in luminal cancers and AR negativity was associated with development of local recurrence in luminal A cancers. However to determine potential role of androgen receptor in followup and treatment of triple negative breast cancers, larger studies with longer follow-ups are required.
Footnotes
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Ethics Committee Approval: The study was evaluated and financially supported by Bezmialem Vakif University, Unit of Scientific Research Project (BAP) with the project number of 6.2011/23. The institute had no objection for conduction of the study by the authors and mentioned that Ethics Comitee Approval was not necessary.
Author Contributions: Concept - M.K.; Design - M.K, Z.G.; Supervision - M.M.; Funding - M.K; Materials - Z.G., U.O.I.; Data Collection and/or Processing - Z.G, U.O.I.; Analysis and/or Interpretation - M.K, F.E.; Literature Review - N.M., F.E.; Writer - M.K, Z.G.; Critical Review - M.M.
Financial Disclosure: The study was financially supported by Bezmialem Vakif University, Unit of Scientific Research Project (BAP) with the project number of 6.2011/23.
Conflict of Interest: No conflict of interest was declared by the authors.
References
- 1.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. http://dx.doi.org/10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, Brufsky AM, Lembersky BC, Ahrendt GM. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy. Cancer. 2010;116:1431–1439. doi: 10.1002/cncr.24876. http://dx.doi.org/10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- 3.Goldhrisch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn H-J Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. http://dx.doi.org/10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. http://dx.doi.org/10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 5.Rakha EA, El-Sayed ME, Green AR, Paish EC, Lee AH, Ellis IO. Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathology. 2007;50:434–438. doi: 10.1111/j.1365-2559.2007.02638.x. http://dx.doi.org/10.1111/j.1365-2559.2007.02638.x. [DOI] [PubMed] [Google Scholar]
- 6.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. http://dx.doi.org/10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. http://dx.doi.org/10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 8.Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J. 2007;21:2285–2293. doi: 10.1096/fj.06-7518com. http://dx.doi.org/10.1096/fj.06-7518com. [DOI] [PubMed] [Google Scholar]
- 9.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. http://dx.doi.org/10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 10.Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. http://dx.doi.org/10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 11.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical and prognostic associations. Am J Clin Pathol. 2003;120:725–731. doi: 10.1309/42F0-0D0D-JD0J-5EDT. http://dx.doi.org/10.1309/42F00D0DJD0J5EDT. [DOI] [PubMed] [Google Scholar]
- 12.Schippinger W, Regitnig P, Dandachi N, Wernecke KD, Bauernhofer T, Samonigg H, Moinfar F. Evaluation of the prognostic significance of androgen receptor expression in metastatic breast cancer. Virchows Arch. 2006;449:24–30. doi: 10.1007/s00428-006-0213-6. http://dx.doi.org/10.1007/s00428-006-0213-6. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, Sakurai K, Inoue T, Nishiguchi Y. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13:431–435. doi: 10.1007/s10147-008-0770-6. http://dx.doi.org/10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. http://dx.doi.org/10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. http://dx.doi.org/10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkilä P, Heikkinen T, Nevanlinna H, Akslen LA, Bégin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, García-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. http://dx.doi.org/10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachapelle J, Foulkes WD. Triple-negative and basal-like breast cancer: implications for oncologists. Curr Oncol. 2011;18:161–164. doi: 10.3747/co.v18i4.824. http://dx.doi.org/10.3747/co.v18i4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. http://dx.doi.org/10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulligan AM, Pinnaduwage D, Bull SB, O’Malley FP, Andrulis IL. Prognostic effect of basal-like breast cancers is time dependent: evidence from tissue microarray studies on a lymph node-negative cohort. Clin Cancer Res. 2008;14:4168–4174. doi: 10.1158/1078-0432.CCR-07-4543. http://dx.doi.org/10.1158/1078-0432.CCR-07-4543. [DOI] [PubMed] [Google Scholar]
- 20.van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Köchli OR, Mross F, Dieterich H, Seitz R, Ross D, Botstein D, Brown P. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161:1991–1996. doi: 10.1016/S0002-9440(10)64476-8. http://dx.doi.org/10.1016/S0002-9440(10)64476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda H, Takarabe T, Hasegawa F, Fukutomi T, Hirohashi S. Large, central acellular zones indicating myoepithelial tumor differentiaton in high-grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol. 2000;24:197–202. doi: 10.1097/00000478-200002000-00005. http://dx.doi.org/10.1097/00000478-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 22.de Azambuja E, Cardoso F, de Castro G, Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. http://dx.doi.org/10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast. 2008;17:323–334. doi: 10.1016/j.breast.2008.02.002. http://dx.doi.org/10.1016/j.breast.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G IMPACT Trialists Group. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. http://dx.doi.org/10.1093/jnci/djm020. [DOI] [PubMed] [Google Scholar]
- 25.Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010;457:467–476. doi: 10.1007/s00428-010-0964-y. http://dx.doi.org/10.1007/s00428-010-0964-y. [DOI] [PubMed] [Google Scholar]
- 26.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD. Androgen receptor inhibits estrogen receptor alfa activity and is prognostic role in breast cancer. Cancer Res. 2009;69:6131–6140. doi: 10.1158/0008-5472.CAN-09-0452. http://dx.doi.org/10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, Traina TA, Hudis C, Hortobagyi GN, Gerald WL, Mills GB, Hennessy BT. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–2478. doi: 10.1158/1078-0432.CCR-08-1763. http://dx.doi.org/10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 28.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G, Sapino A. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124:607–617. doi: 10.1007/s10549-010-0761-y. http://dx.doi.org/10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21:488–492. doi: 10.1093/annonc/mdp510. http://dx.doi.org/10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 30.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–131. doi: 10.1016/j.ccr.2011.05.026. http://dx.doi.org/10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshadri R, Shah PN. Steroid receptors in breast cancer in Indian women. Anticancer Res. 1984;4:403–407. [PubMed] [Google Scholar]


