Abstract
Objectives:
In 2010, South Africa reported an early mother-to-child transmission (MTCT) rate of 3.5% at 4–8 weeks postpartum. Provincial early MTCT rates ranged from 1.4% [95% confidence interval (CI): 0.1 to 3.4] to 5.9% (95% CI: 3.8 to 8.0). We sought to determine reasons for these geographic differences in MTCT rates.
Methods:
This study used multilevel modeling using 2010 South African prevention of mother-to-child transmission (PMTCT) evaluation (SAPMTCTE) data from 530 facilities. Interview data and blood samples of infants were collected from 3085 mother–infant pairs at 4–8 weeks postpartum. Facility-level data on human resources, referral systems, linkages to care, and record keeping were collected through facility staff interviews. Provincial level data were gathered from publicly available data (eg, health professionals per 10,000 population) or aggregated at province-level from the SAPMTCTE (PMTCT maternal-infant antiretroviral (ARV) coverage). Variance partition coefficients and odds ratios (for provincial facility- and individual-level factors influencing MTCT) from multilevel modeling are reported.
Results:
The provincial- (5.0%) and facility-level (1.4%) variance partition coefficients showed no substantive geographic variation in early MTCT. In multivariable analysis accounting for the multilevel nature of the data, the following were associated with early MTCT: individual-level—low maternal–infant ARV uptake [adjusted odds ratio (AOR) = 2.5, 95% CI: 1.7 to 3.5], mixed breastfeeding (AOR = 1.9, 95% CI: 1.3 to 2.9) and maternal age <20 years (AOR 1.8, 95% CI: 1.1 to 3.0); facility-level–insufficient (≤2) health care-personnel for HIV-testing services (AOR = 1.8, 95% CI: 1.1 to 3.0); provincial-level PMTCT ARV (maternal–infant) coverage lower than 80% (AOR = 1.4, 95% CI: 1.1 to 1.9), and number of health professionals per 10,000 population (AOR = 0.99, 95% CI: 0.98 to 0.99).
Conclusions:
There was no substantial province-/facility-level MTCT difference. This could be due to good overall performance in reducing early MTCT. Disparities in human resource allocation (including allocation of insufficient health care personnel for testing and care at facility level) and PMTCT coverage influenced overall PMTCT programme performance. These are long-standing systemic problems that impact quality of care.
Key Words: population-level variations, mother-to-child transmission of HIV, PMTCT, universal access
INTRODUCTION
By mid-2014, an estimated 170,000 children in low- and middle-income countries were infected annually with HIV through mother-to-child HIV transmission (MTCT).1 The World Health Organization outlines a case rate of new pediatric HIV infections ≤50 per 100,000 livebirths as one of the minimum criteria for validating elimination of MTCT of HIV among children.2 Sub-Saharan African countries have shown substantial progress in reducing MTCT rates, but achievements vary considerably across populations.3 In South Africa, though the national early (4–8 weeks postpartum) transmission rate from 2 consecutive national surveys (2010, 2011) is reported at 3.5% [95% confidence interval (CI): 2.9 to 4.1] and 2.7% (95% CI: 2.1 to 3.2), respectively, transmission rates vary between provinces ranging from 1.4%–5.9% in 2010 and 2.0%–6.1% in 2011.4,5 What explains the variation in transmission rates across provinces is not well understood.
Studies show that the South African health system suffers from historical inequity in health resource allocation among and within provinces.6 Trends in provincial and local government primary health care (PHC) expenditure per capita (uninsured population) for the years 2011/2012–2014/2015 show an annual minimum 30% difference between the province with the lowest PHC expenditure per capita and the province with the highest.7–9 Limited research has been done to assess whether the disparity in resource allocation between provinces, and other facility- and provincial-level factors, has any impact on the performance of the prevention of mother-to-child transmission of HIV (PMTCT) programmes across and within provinces.10
This article aims to understand the contribution of factors at 3 levels, namely, individual, facility, and provincial, to the early (4–8 weeks postpartum) MTCT measured in each province of South Africa using data from 2010 South African PMTCT Evaluation (SAPMTCTE).
METHODS
Study Design, Sample Size, Sampling
The detailed methods used in this study have been described elsewhere.11 In brief, the SAPMTCTE, from which the early MTCT and individual-level factors have been obtained, was based on a complex survey design. The total population was first stratified by province (n = 9) and within each province, public PHC clinics and community health centers (CHCs) were stratified into 3 groups based on their 6-week annual immunization numbers (extracted from the 2007 district health information system data) and antenatal HIV prevalence of the district (from 2009 antenatal survey): small [<130 annual diphtheria–tetanus–pertussis−1 (DTP1) coverage], medium (130–300 annual DTP1 coverage), large (≥300 annual DTP1 coverage) with below the 2009 national average antenatal HIV prevalence (<29%), and large with above the 2009 national average antenatal HIV prevalence (≥29%).12 This was followed by a selection of facilities using probability proportional to size (with replacement) sampling methods. At the final step, a fixed number of individual mother–infant pairs attending 6-week immunization visits were recruited consecutively or systematically from each selected facility within a specified period. Individual mother–infant pairs represent the lower-level (level 1) units, who are nested within health facilities (level 2) and health facilities are nested within provinces (level 3) providing a natural 3-level hierarchy.
A detailed description of sample size is presented elsewhere.4 In summary, the sample size calculation was targeted to provide national and provincial level stable estimates of transmission rates. The following indicators were taken into account in sample-size calculation: the 2009 antenatal HIV prevalence,13 transmission rate estimates from 2 previous regional surveys,14,15 and the coverage of PMTCT antiretroviral (ARV) prophylaxis in each province from district health information system reports, with varying MTCT precision levels by province (ranging from 1% to 2%) and a design effect of 2 to account for clustering within health facilities. Based on this, a desired sample size [ie, the collection of interview data and dried blood spots (DBSs) of infants] of 12,200 mother–infant pairs from 580 facilities was needed.
Data Collection
Individual-Level Indicators
Mother/caregiver–infant pairs visiting 6-week immunization services at the selected 580 facilities were approached and screened for eligibility by trained nurses. Mothers/caregivers were recruited if their infants were 4–8 weeks old, were receiving DPT1 vaccination that day, had no emergency illness, and mother/caregiver consented to participate in the study. Those who gave consent were interviewed on antenatal and peripartum services received, socio-demographic indicators, and knowledge about PMTCT and PMTCT services received. The infant Road-To-Health-Card was checked for documentation of maternal and infant HIV status, gestational age at birth, and birth weight. We spent 3 weeks in 8 provinces and 4 weeks in 1 province (Northern Cape) in each facility for data collection. Interview data were collected on hand-held devices (cell phone preprogrammed with a questionnaire).
After the interview, individual pretest counseling was given to each mother and if mothers consented, DBSs of infants were collected using heel-prick. Blood specimens of infants were collected from all infants irrespective of prior knowledge of the HIV-exposure status of the infant. DBS specimens were tested for HIV antibodies by means of an enzyme immunoassay (EIA) (Genscreen HIV1/2 Ab EIA Version 2; Bio-Rad Laboratories, Schiltigheim, France). A positive EIA result indicated infant HIV-exposure and was considered a proxy indicator for maternal HIV-positive status. A qualitative HIV polymerase chain reaction (PCR) test [COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) Qualitative assay version 1.0 assay; Roche Diagnostics, Branchburg, NJ] was performed on all EIA-positive DBS, and DBS of infants born to self-reported HIV-positive women to determine whether the infant was HIV PCR positive (ie, HIV-infected).
Health Facility-Level Indicators
Data on health facility-level indicators were collected from 530 of the selected 580 facilities after a situational assessment conducted 6 months before the SAPMTCTE. Trained field workers used open-ended and close-ended questions to collect data on human resources for health (HRH), referral systems, record keeping, linkages, and organization of systems for PMTCT during structured interviews with clinic managers, district health information officers, immunization nurses, PMTCT nurses, and sick-child (integrated management of childhood illnesses) nurses.
Provincial Level Indicators
Provincial level indicators were obtained from publicly available reports from the National Treasury South Africa [on proportion of total provincial budget allocated to the Department of Health (DoH) and annual under spending of health budget],16–18 Statistics South Africa (on provincial proportion whose income or consumption is below the upper-bound poverty line and proportion of children living in rural areas19,20). Aggregated data were extracted from National DOH (NDOH) Antenatal Survey report (provincial HIV prevalence among pregnant women),13 the South African Health Review,21 and the 2010 District Health Barometer (proportion of under 18 facility deliveries, PHC expenditure per capita and percent of expenditure on PHC facilities).22 Provincial health HRH data (total health professionals per 10,000 population) for 2010 were drawn from the NDoH Draft HRH Consultation and Strategy Document.23 All provincial data gathered were for the year 2009/2010. Provincial perinatal PMTCT ARV regimen coverage and HIV transmission rates were aggregated from the current (SAPMTCTE) data.
Statistical Analysis
All HIV-exposed infants with individual- (interview and PCR-negative or PCR-positive test results), facility- and provincial-level information were included in this analysis. We used percentages, medians, and inter-quartile ranges (IQRs) to describe data at individual-, health facility-, and provincial-level. PMTCT ARV regimen coverage (at province-level) was defined as the proportion of HIV-positive mothers (identified by reactive infant EIA test) who received any maternal antiretroviral prophylaxis or treatment (cART) with infant nevirapine (NVP)/azidothymidine. The United Nations General Assembly (UNGASS) universal PMTCT coverage goal of ≥80% was used as the cut-off for “good” PMTCT ARV coverage. Based on this, provincial PMTCT ARV coverage was categorized into 2 categories: below 80% PMTCT ARV coverage and ≥80% PMTCT ARV coverage. A socio-economic score was created based on the availability of assets (television, car, refrigerator, stove), and dwelling characteristics (type of water source, toilet, fuel and building material) using the principal component analysis method. Provinces were ranked 1–9 according to their performance on each of the following 4 indicators: PMTCT ARV coverage, HRH (ie, number of health professionals per 10,000 population), budget (proportion of provincial budget allocated for DoH), and poverty measure (proportion below the upper-bound poverty line).
We used multilevel mixed (MLM) effects logistic regression models with random facility-level and provincial-level intercepts to examine correlates of MTCT at individual-, facility-, and provincial-level. The multilevel analysis was implemented in a stepwise manner starting with the unconditional model (null model) which was fitted to determine the significance of the 2 random effects (facility and province) and the intraclass correlation coefficient that describes the proportion of variance that is attributable to clustering at facility- and provincial-level. We used a likelihood ratio test to compare the null MLM model with a single level (ie, no intercept) logit model to determine the significance of the facility and province random intercepts. Three models were subsequently fitted by including (into the null model) individual-level factors (model 1), followed by health facility-level factors (model 2), and provincial-level factors (model 3). Individual-, facility- and provincial-level indicators were included in the model if their P value in a bivariate analysis was below 0.2.
The MLM models were weighted at both facility- (level 2) and individual-levels (level 1). The level 1 weights were computed as the product of the population size (births) and the sample size realization weights.24 There was no design weight at level 1 as we did a period census in each facility and all mothers eligible up to the required sample size were included. The weight for level 2 (health facility-level) was calculated for each facility as the inverse of the sampling probability which was calculated taking into account the probability proportional to size sampling method. Provinces (level 3) were selected with full certainty so the weight for level 3 was equal to that of 1. For the MLM models, level 1 and level 2 weights were scaled using one of the methods discussed by Pfeffermann et al.25 The method used here is to scale the weights so that their sum equals to the effective sample size. This method improves estimation of variance components when both level 1 and level 2 samplings are noninformative as is the case in our study. Descriptive analysis incorporated either facility-level nonscaled weights (for facility-level variables) or both facility- and individual-level nonscaled weights (for individual-level variables and for provincial PMTCT ARV regimen coverage).
From individual-level variables, gestational age at birth had the highest (23%) missing responses, and of the facility-level variables, 10%–20% of data were missing on a number of variables. To account for uncertainty arising from missing data, we performed multiple imputations using a multilevel random effects multiple imputation model, REALCOM Impute package (developed by Harvey Goldstein at the Centre for Multilevel Modeling in Bristol). We had no missing data for level 3 variables, therefore imputation was only performed for relevant individual- and facility-level variables. Information across levels was used to improve the quality of imputation.
Variance coefficients from multiple imputed data sets were combined using Rubin's rule.26 The variance partition coefficient (VPC) was calculated as a proportion of total variance explained by facility- and provincial-level random effects, respectively. The median odds ratio (MOR) which quantifies the between cluster (ie, province and facility) variance by comparing 2 persons from different clusters is reported. All analyses were done using STATA SE (version 12; StataCorp LP, College Station, TX).
The survey protocol was approved by the institutional review board of the South African Medical Research Council and the Office of the Associate Director of Science at the United States Centers for Disease Control and Prevention. The South African Medical Research Council Ethics Committee approved the situational assessment protocol (Ref: EC09-002). Informed consent was obtained verbally from all participating clinic nurses/managers, and written consent from participating mothers/caregivers.
RESULTS
Study Sample
The 2010 national SAPMTCTE achieved 83.4% (10,178) of the planned 12,200 sample size: 3 provinces achieved less than 75% of the target sample size, namely, Limpopo (LP) (73%), Eastern Cape (EC) (55%), and Northern Cape (NC) (63%). Out of the 10,178 enrolled study participants (infants) 3088 were HIV-exposed; 3085 of these infants, from 530 facilities, with both individual- (interview and PCR-negative or PCR-positive results) and facility-level data were included in this analysis.
Individual-Level Data
Most mothers included in this analysis were single (79.0%), from Black/African race (98.6%), with some secondary-level education (76.4%). The median maternal age was 28 years (IQR: 24–32). Less than a fifth (16.8%) of infants had low birth weight (<2.5 kg) (Table 1).
TABLE 1.
Individual- and Facility-Level Characteristics of the Study Sample
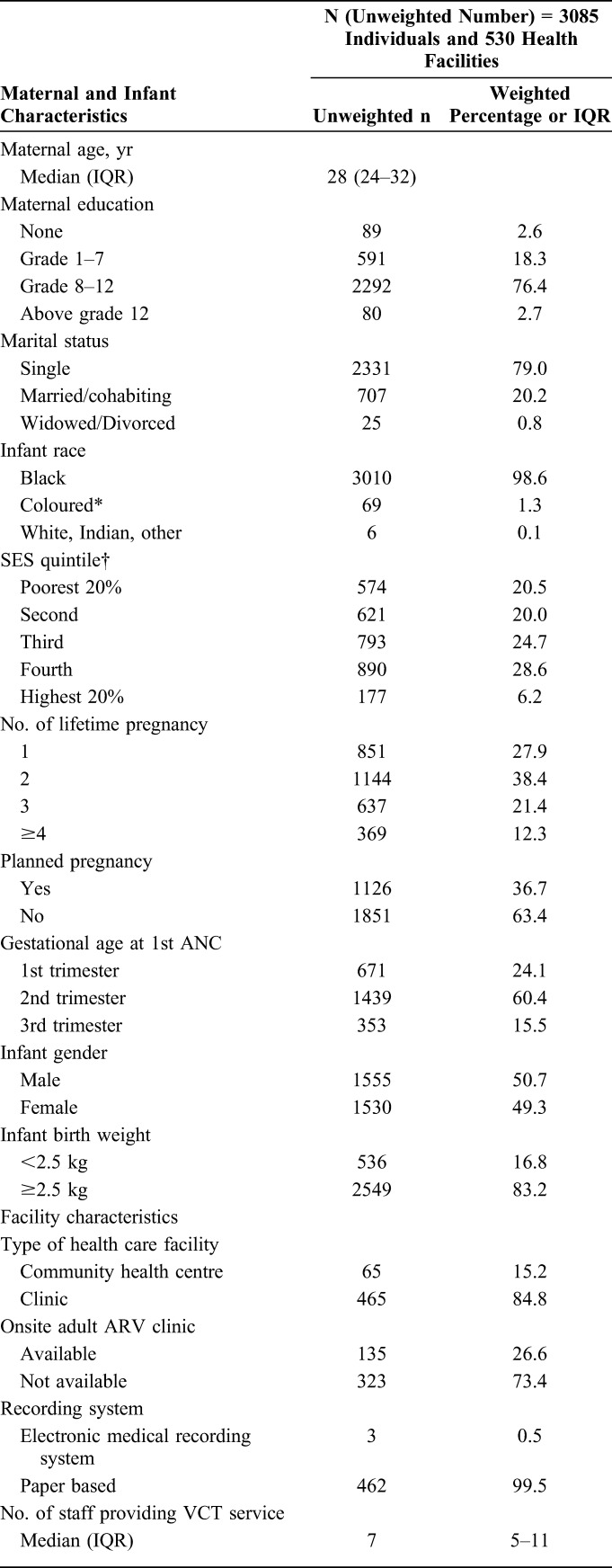
Health Facility-Level Data
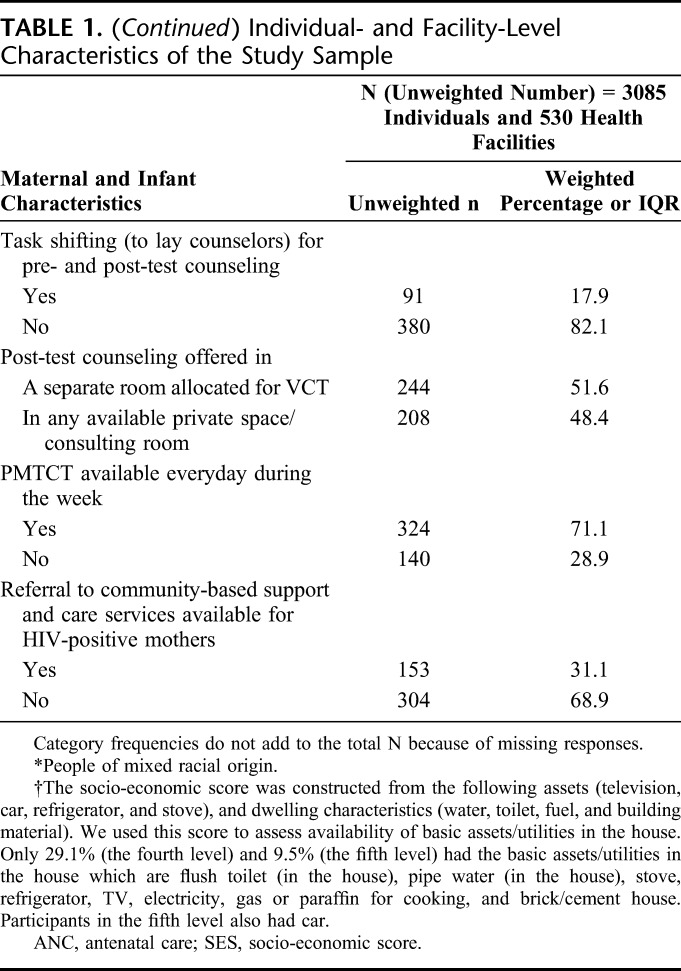
Of the facilities visited 15.2% (65) were community health centers and 84.8% (465) were PHC Clinics. Most (71.1%) offered daily PMTCT services. In each facility, a median number of 7 (IQR 5–11) staff was allocated to provide HIV testing services (HTSs); 17.9% reported that the task of pre- and post-HIV–test counseling had shifted to lay counselors. Just more than half (51.6%) of selected facilities reported having a separate room allocated for post-test counseling of mothers, and the rest 48.4% reported that post-test counseling was provided in any available private space/consulting room (Table 1).
Provincial-Level Indicators
Among HIV-positive (infant EIA positive) mothers, coverage of PMTCT ARV regimen (ie, either dual prophylaxis or cART plus infant NVP at birth) varied by province ranging from 63.9% in EC to 87.4% in KwaZulu-Natal (KZN). Three provinces namely, KZN, Gauteng (GP), and Western Cape (WC) achieved the UNGASS target for universal access (≥80.0%) to PMTCT ARV regimens (range 80.1%–87.4%). Mpumalanga (MP), LP, and EC had the lowest (range 63.9%–66.0%) PMTCT ARV coverage (Table 2).
TABLE 2.
Provincial-Level Comparison of Early Transmission Rates With ARV Regimen Coverage, Health Professionals and Budget Distribution, and Population Socioeconomic Status
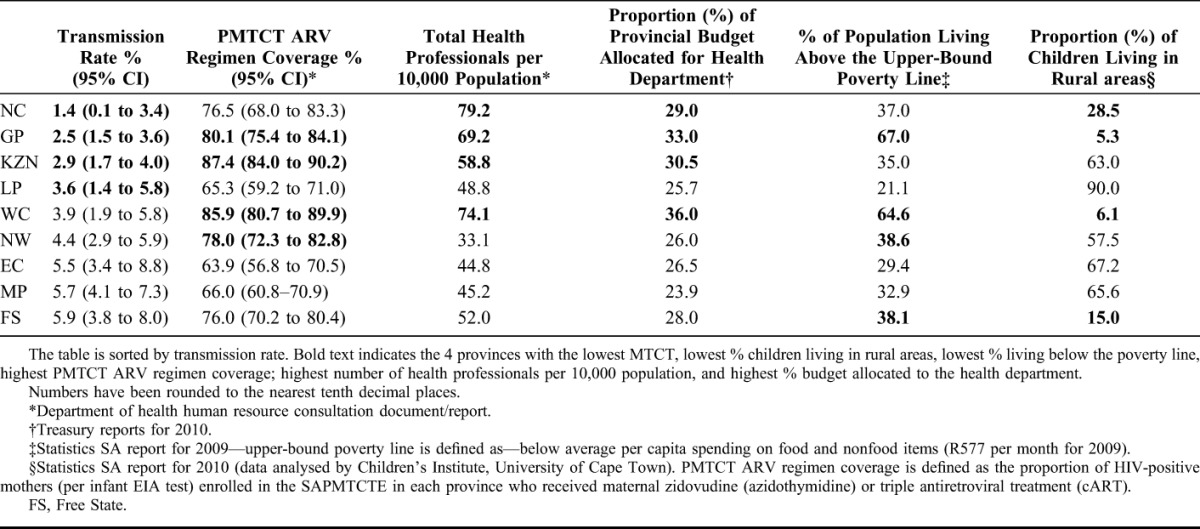
HRH distribution across provinces ranged from 33 health professionals per 10,000 total population in North West (NW) to >70 per 10,000 population in NC and WC provinces. Proportion of total provincial budget allocated for health was highest in WC (36.0%) and GP (33.0%) and lowest in MP (23.9%). In terms of socioeconomic indicators, most (>60%) GP and WC population were living above the upper-bound poverty line and were urbanized (with <10.0% rural population) compared with the other 7 provinces (Table 2). When provinces were ranked according to their performance on PMTCT ARV coverage, budget allocation, poverty measure, and HRH indicators, WC and GP overall ranked as the best performing provinces, whereas LP, EC, and MP ranked as the least well-performing provinces.
Multilevel Mixed Effect Model With Random Effects Only (Null Model)
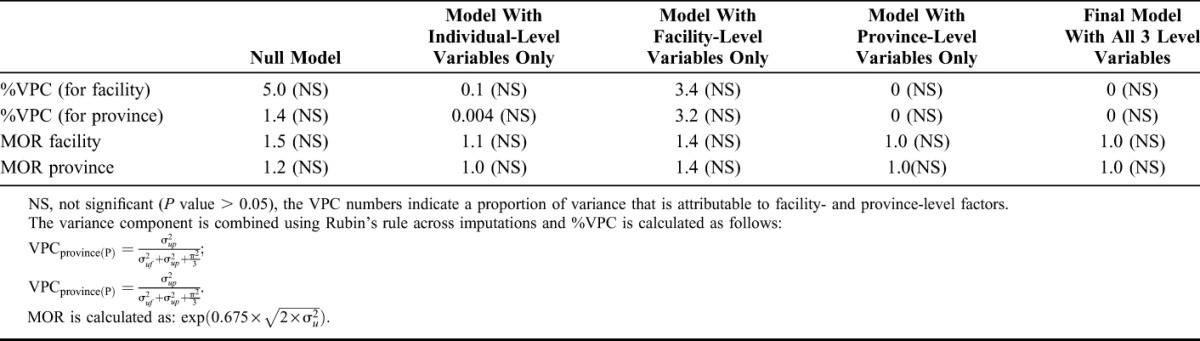
When the multilevel (3 level) model for MTCT was compared with the single-level (null) logistic regression model for MTCT, no significant (P value = 0.26) differences were found, showing that MTCT is not structurally correlated at the hierarchical level of province and facility (Table 3). Only a small proportion of the variance in early MTCT was attributable to the differences seen in MTCT rates across facilities (VPC = 5.0%; MOR = 1.5) and provinces (VPC = 1.4%; MOR = 1.2) (Table 4).
TABLE 3.
Comparison of Multilevel Null Model With Single-Level (Null) Logistic Model
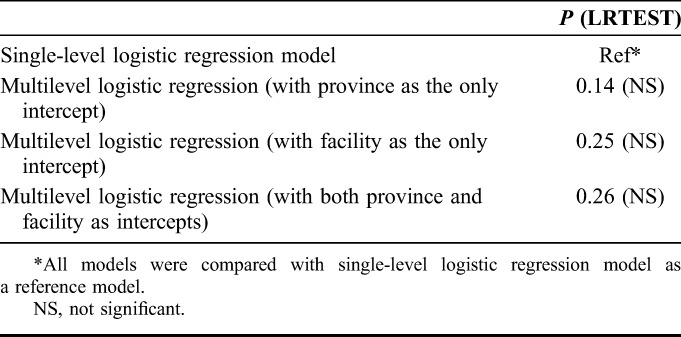
TABLE 4:
Random Effects Result
Bivariable and Multivariable Multilevel Models
In bivariable and multivariable multilevel modelling, 3 individual-level indicators were significant predictors of early MTCT, namely, low uptake of maternal, infant, or both ARVs (dual prophylaxis or cART plus infant NVP) [adjusted odds ratio (AOR) 2.5, 95% CI: 1.7 to 3.5], feeding pattern (mixed breastfeeding vs other) (AOR 1.9, 95% CI: 1.3 to 2.9) and maternal age below 20 years (AOR 1.8, 95% CI: 1.1 to 3.0) (Table 5). The odds ratio for the relationship between feeding pattern and MTCT, and age <20 years and MTCT was reduced when controlling for facility-level factors, and the odds ratio between ARV coverage and MTCT reduced when controlling for provincial level factors (Table 5).
TABLE 5.
Crude and Adjusted Odds Ratios (OR) for Individual-, Facility-, and Provincial-Level Factors Associated With Infant HIV Transmission Among 4–8 Weeks Old HIV-Exposed Infants in the SAPMTCTE
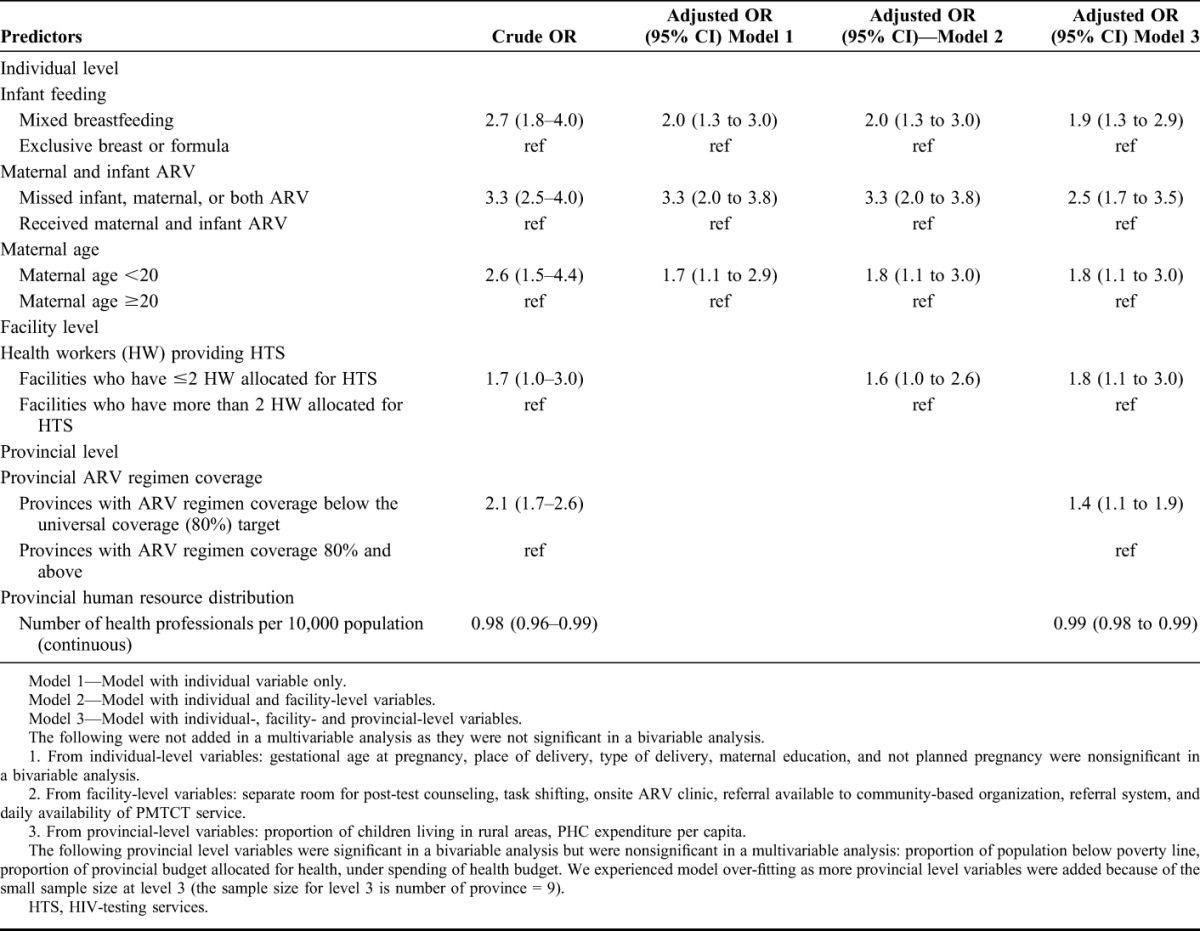
Of the facility-level indicators, in the unadjusted model, the number of health professionals allocated for HTS was an influential predictor of early MTCT. After adjusting for individual- and provincial-level factors, facilities that allocated ≤2 staff for HTS had a significantly higher odds of transmission (AOR 1.8, 95% CI: 1.1 to 3.0) compared with those who allocated more than 2 health personnel for providing HTS. Other facility-level variables (ie, indicators for referral system, record keeping, and infrastructure) were nonsignificant in both bivariable and multivariable multilevel modelling (Table 5).
From provincial-level indicators, in adjusted models, provinces with lower than universal (80.0%) coverage for perinatal PMTCT ARV regimens had significantly higher (AOR 1.4, 95% CI: 1.1 to 1.9) MTCT compared with provinces that achieved universal coverage (Table 5). Provincial variation in HRH distribution was also a significant predictor of MTCT in adjusted models—for each additional health professional per 10,000 population, MTCT decreased by 0.01% (AOR 0.99, 95% CI: 0.98 to 0.997) (Table 5). Other provincial indicators, such as provincial budget allocation for health and poverty measures were significantly associated with transmission only in a bivariable analysis and were nonsignificant in a multivariable analysis.
DISCUSSION
This study shows no substantive geographic (province-/facility-level) differences in early MTCT. Although early MTCT was associated with both individual and aggregate/contextual (facility- and provincial-level) factors, the overall contribution of aggregate level factors to early MTCT was modest (provincial VPC = 1.4% and facility VPC = 5%). The lack of significant geographic variation in MTCT could be due to good overall performance across provinces and facilities in reducing perinatal MTCT.
Despite a moderate effect on MTCT, some of the aggregate-level factors identified in this study are long-standing problems of the health care–system in South Africa with reported serious impact on quality of care.10,27,28 Inequitable HRH distribution—one of the provincial level factors identified as influential predictor of MTCT in this study has been reported as a primary bottleneck for delivering quality health care in South Africa.10,27,28 At facility level, we found allocation of ≤2 staff for HIV-testing services as a significant risk factor for MTCT. In the new South African national HTS guideline released in 2016, HTS staff are tasked with a number of responsibilities including providing pretest information, HIV testing, posttest counseling, and active referral of HIV-positive clients to ART clinic: recommended referral methods include escorting HIV-positive clients to ARV clinics (if within same facility) or setting-up appointment at the receiving facility (if ART is not provided within the same facility).29 While these services are important, facilities with only 2 staff allocated for HTS could struggle to provide these services at an acceptable level of quality. More health workers, with the right mix of skills, are needed to provide HIV services that are at acceptable standard. We recommend implementing effective recruitment and retention strategies (including appropriate selection of students and training of health professionals in rural areas, financial incentives, and capacity building support), task shifting, introducing patient appointment system, and decentralization of service to lower-level care to redress the inequitable distribution and inadequate staffing of PHC facilties.27,30,31 The provinces with the least HRH allocation (NW, EC, MP, and LP) need to be prioritized in redressing the inequitable distribution of HRH. In addition, the disparities in HRH within province (eg, between rural and urban facilities) should be addressed.
The provinces that achieved the UNGASS PMTCT ARV regimen target (≥80% coverage) had a significantly lower MTCT rate compared with provinces that did not achieve the UNGASS target. Three of the provinces in this study achieved at least 80% ARV coverage—early MTCT in 2 of the 3 provinces was ≤2.5%. This low MTCT levels achieved with PMTCT coverages of 80% and above show promise for targets to eliminate MTCT by 2020.
The study limitations are acknowledged. The lack of significant variability at province- and facility-level could be a result of 2 features: first, the small number of infections overall; and second, the even smaller number of infections at the facility-level. Given that the prevalence of our outcome measure (ie, MTCT) is small, a large sample size would be needed at level 1 (individual-level) to precisely measure provincial- and facility-level HIV transmission rates. In our study, the sample size achievement at province-level was below the required sample size, with 3 of 9 provinces achieving below 75% of the required sample size. As a result, although the MTCT point estimates varied across provinces (ranging between 1.4% and 5.9%), the CIs around these estimates were fairly wide implying unstable estimates or large variance of the provincial MTCT point estimates. As South Africa contains only 9 provinces, the available observations at level 3 (province-level) in our study were also slightly fewer than the recommended minimum sample size limit—a minimum of 10–30 sample size is recommended (at level 3) for multilevel modeling whereas our sample size (n = 9) was close to the lower limit (10).32
In conclusion, in this study, there were no substantial province-/facility-level MTCT differences because of good overall performance in reducing early MTCT. However, facility- and provincial-level factors play a role in the relationship between individual-level factors and MTCT. Most of the facility-/province-level factors examined (such as human resource) are long-standing problems of the health care–system in South Africa. Plans to improve overall maternal and child health outcome indicators should aim to address these aggregate as well as individual-level factors. This includes continued investment in human resource management and planning, and improving the overall provincial achievement in PMTCT ARV coverage.
Footnotes
Supported by the President's Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of Cooperative Agreements 1U2GPS001137-02 and 1U2GPS001137-03, the National Department of Health, United Nations Children's Fund, The National Research Foundation, the Clinton Health Access Initiative, African Doctoral Dissertation Research Fellowship, and South African Centre for Epidemiological Modeling and Analysis.
The authors have no conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control.
REFERENCES
- 1.UNAIDS. 2015 Progress Report on the Global Plan. Available at: http://www.unaids.org/en/resources/documents/2015/JC2774_2015ProgressReport_GlobalPlan. Accessed December 23, 2015. [Google Scholar]
- 2.WHO. Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission of HIV and Syphilis. 2014. Available at: http://www.who.int/reproductivehealth/publications/rtis/9789241505888/en/. Accessed September 22, 2015. [Google Scholar]
- 3.Global report. UNAIDS Report on the Global AIDS Epidemic 2013. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf. Accessed September 17, 2015. [Google Scholar]
- 4.Goga A, Dinh T, Jackson D; for the SAPMTCTE Study Group. Evaluation of the Effectiveness of the National Prevention of Mother-to-Child Transmission (Pmtct) Programme Measured at Six Weeks Postpartum in South Africa. 2010. Available at: http://www.info.gov.za/view/DownloadFileAction?id=165280. Accessed August 17, 2012. [Google Scholar]
- 5.Goga A, Dinh T, Jackson D. 2011 SAPMTCTE Report Early (4-8 Weeks Post-Delivery) Population-Level Effectiveness of WHO PMTCT Option A, South Africa. 2011. Available at: www.mrc.ac.za/healthsystems/SAPMTCTE2011.pdf. Accessed August 17, 2014. [Google Scholar]
- 6.McIntyre D, Muirhead D, Gilson L. Geographic patterns of deprivation in South Africa: informing health equity analyses and public resource allocation strategies. Health Pol Plann. 2002(suppl 17):30–39. [DOI] [PubMed] [Google Scholar]
- 7.Massyn N, Day C, Peer N, et al. District Health Barometer 2013/14. Durban, South Africa: Health system unit; 2014. [Google Scholar]
- 8.Massyn N, Day C, Peer N, et al. District Health Barometer 2014/15. Durban, South Africa: Health system unit; 2015. [Google Scholar]
- 9.Massyn N, Day C, Peer N, et al. District Health Barometer 2012/13. Durban, South Africa: Health system unit; 2013. [Google Scholar]
- 10.Lerebo W, Callens S, Jackson D, et al. Identifying factors associated with the uptake of prevention of mother to child HIV transmission programme in Tigray region, Ethiopia: a multilevel modeling approach. BMC Health Serv Res. 2014;14:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goga AE, Dinh TH, Jackson DJ, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health. 2014:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DHIS. District Health Information System. 2007. Available at: http://www.hst.org.za/publications/841. Accessed January 22, 2011. [Google Scholar]
- 13.National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa. Pretoria, South Africa: Department of Health; 2009: Available at: http://www.health-e.org.za/documents/85d3dad6136e8ca9d02cceb7f4a36145.pdf. Accessed October 14, 2012. [Google Scholar]
- 14.Rollins N, Little K, Mzolo S, et al. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–1347. [DOI] [PubMed] [Google Scholar]
- 15.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. [DOI] [PubMed] [Google Scholar]
- 16.National Treasury. Estimates of Provincial Revenue and Expenditure (EPRE), 2007/08, 2008/09, 2009/10, 2010/11, & 2011/12. Available at: www.treasury.gov.za. Accessed April 3, 2014. [Google Scholar]
- 17.Provincial Budgets and Expenditure Review: 2005/2006—2011/2012. Available at: http://www.gov.za/provincial-budgets-and-expenditure-review. Accessed 23 January, 2015. [Google Scholar]
- 18.Lomahoza K, Brockerhoff S, Frye I. A Review of National And Provincial Government Budgets in South Africa 2007/08–2011/12, Monitoring the Progressive Realisation of Socio Economic Rights Project, Working Paper 7. Studies in Poverty and Inequality Institute (SPII); 2013. [Google Scholar]
- 19.Poverty Trends in South Africa an Examination of Absolute Poverty Between 2006 And 2011/Statistics South Africa. Statistics South Africa; 2014. [Google Scholar]
- 20.Katharine H, Winnie S. Housing and Services—Urban-Rural Distribution. Children's Institute, University of Cape Town; Cape Town, South Africa: University of Cape Town; 2014. [Google Scholar]
- 21.Day C, Gray A. South African health review 2012/13, health and related indicators. Available at: http://www.hst.org.za/publications/south-African-health-review-2012/2013. Accessed September 27, 2015.
- 22.HST. District Health Information System (DHIS). Available at: http://www.hst.org.za/district-health-barometer-dhb-22010. Accessed April 28, 2015. [Google Scholar]
- 23.Department of Health Human Resource for Health Consultation Document v5. 2011. Available at: http://www.gov.za/sites/www.gov.za/files/DoH_draftHRstrategy_Consultation.pdf. Accessed September 2, 2015.
- 24.Carle A. Fitting multilevel models in complex survey data with design weights: Recommendations. BMC Med Res Methodol. 2009;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeffermann D, Skinner CJ, Holmes DJ, et al. Weighting for unequal selection probabilities in multilevel models. JRSS. 1998;60(series B):123–140. [Google Scholar]
- 26.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- 27.Chan A, Ford D, Namata H, et al. The Lablite project: a cross-sectional mapping survey of decentralized HIV service provision in Malawi, Uganda and Zimbabwe. BMC Health Serv Res. 2014;14:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eygelaar J, Stellenberg E. Barriers to quality patient care in rural district hospitals. Curationis. 2012;34:8. [DOI] [PubMed] [Google Scholar]
- 29.NDOH. National HIV Testing Services: Policy and Guidelines. Pretoria, South Africa: Department of Health; 2016. [Google Scholar]
- 30.Homsy J, Kalamya J, Obonyo J, et al. Routine intrapartum HIV counseling and testing for prevention of mother-to-child transmission of HIV in a rural Ugandan hospital. J Acquir Immune Defic Syndr. 2006;42:149–154. [DOI] [PubMed] [Google Scholar]
- 31.Versteeg M, du Toit L, Couper I. Building consensus on key priorities for rural health care in South Africa using the Delphi technique. Glob Health Action. 2013;6:19522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryan M, Jenkins S. Regression analysis of country effects using multilevel data: a cautionary tale. IZA Discussion Paper No. 7583. Available at SSRN: http://ssrn.com/abstract=2322088. 2013. Accessed September 21, 2015.


