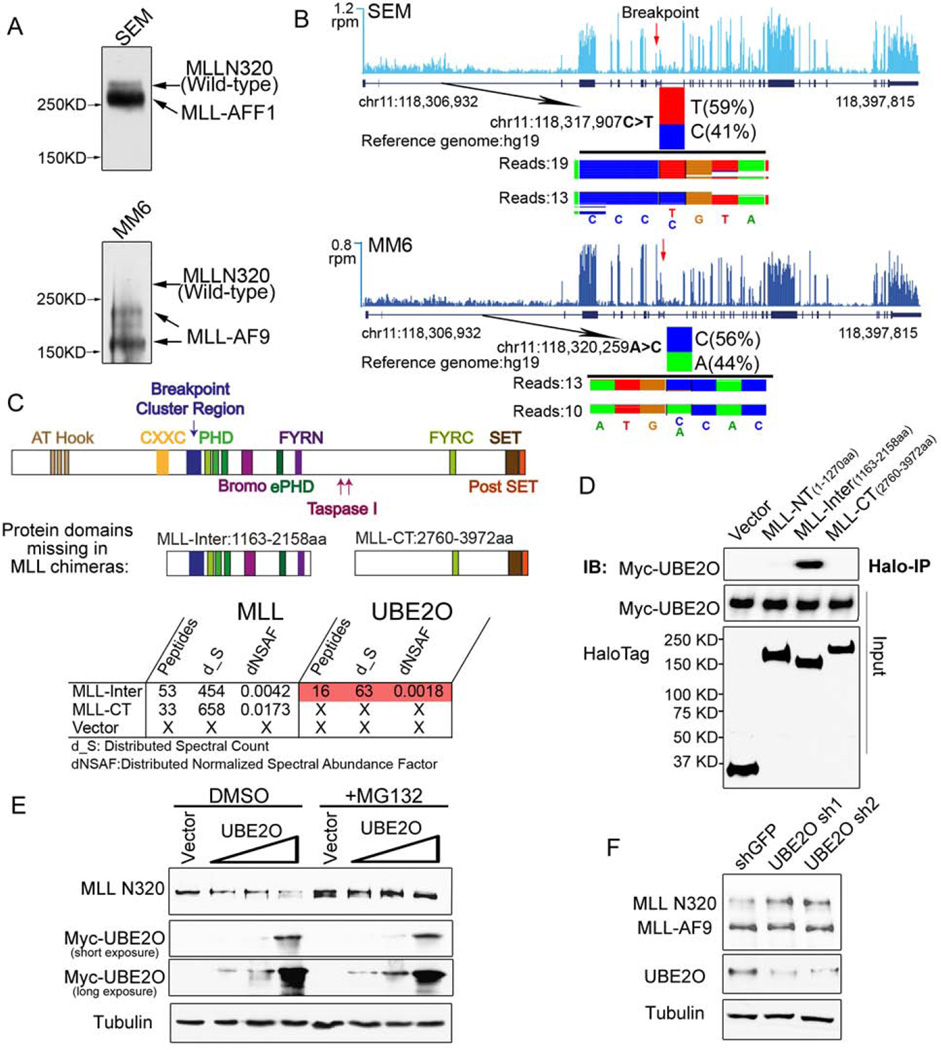
Figure 1. UBE2O interacts with an MLL-internal region and promotes wild-type MLL protein degradation.
(A) MLL-rearranged leukemia cell lines SEM (MLL-AFF1), MM6 (MLL-AF9) have relatively low levels of wild-type MLL protein compared to the MLL chimeras. Immunoblotting of MLL N320 with the D2M7U monoclonal antibody was performed with total cell lysates of SEM and MM6 cells.
(B) Wild-type MLL and MLL fusion alleles are transcribed at similar levels in SEM and MM6 cells. Total RNA-seq was performed with SEM and MM6 cells. Genome browser tracks of MLL RNA are shown, with reads per million (r.p.m.) indicated on the y-axis. The single nucleotide polymorphisms N-terminal to the breakpoint of the MLL gene at chr11:118,317,907 (C>T) seen in SEM cells and at chr11:118,320,259 (A>C) in MM6 cells are shown below each track, with the number of sequencing reads from each allele indicated.
(C–D) Identification of UBE2O as a MLL-Inter specific interacting protein. Halo-tagged MLL-internal region (MLL-Inter) and C-terminal region (MLL-CT), both of which are missing in the MLL chimeras, were transiently transfected into HEK293 cells. MudPIT analysis identified the UBE2O as the most abundant protein specifically interacting with MLL-Inter (Table S1). Interaction between UBE2O and MLL-Inter was confirmed by co-immunoprecipitation (D).
(E) Ectopic expression of UBE2O induces MLL degradation. HEK293 cells were transfected with increasing amounts of Myc-UBE2O expression plasmid. 24 h later, cells were treated with DMSO or MG132 for 12 h.
(F) Depletion of UBE2O specifically stabilizes wild-type MLL, but not the MLL chimera MLL-AF9. UBE2O was depleted with two independent shRNAs in Flag-MLL-AF9 HEK293 cells. 4 days after infection, the MLL N320 and MLL-AF9 levels were determined with the D2M7U antibody.
See also Figures S1 and Table S1.