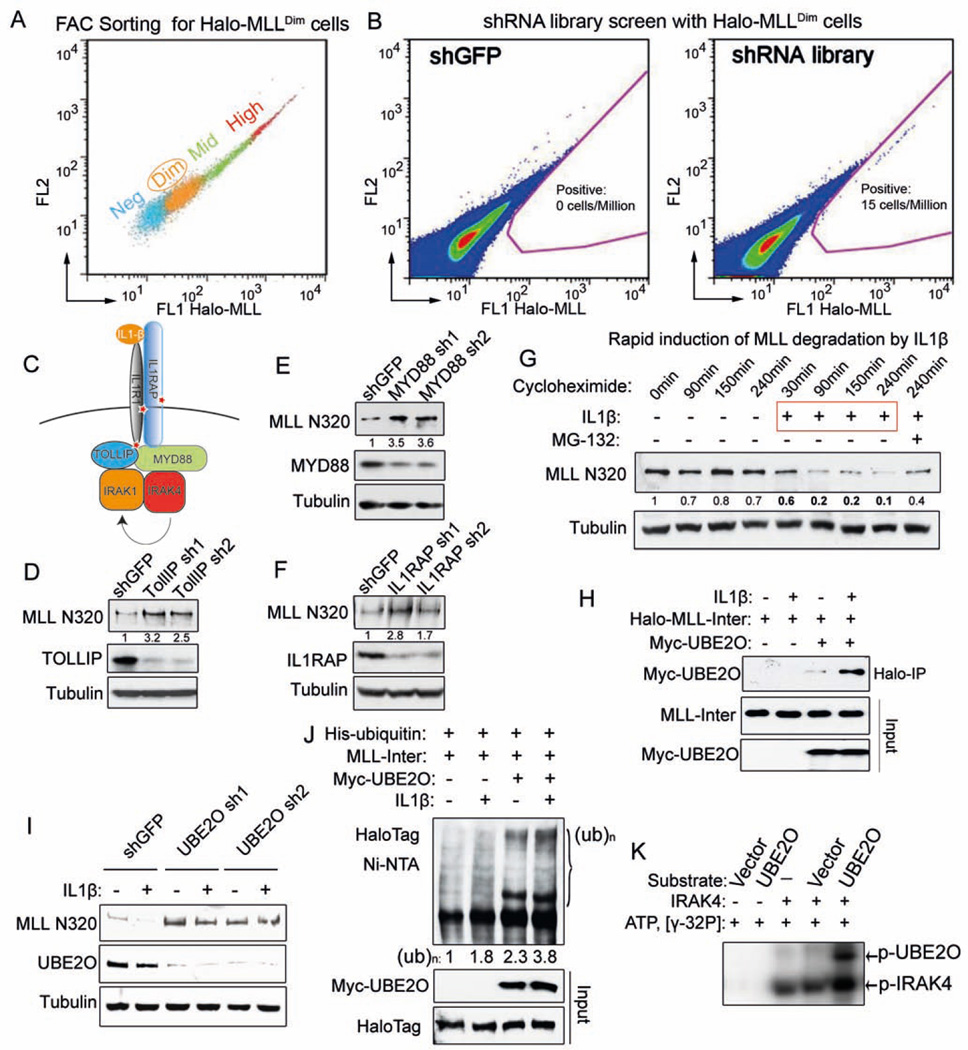
Figure 2. Genome-wide shRNA screen identifies the IL-1 pathway in promoting MLL degradation through an MLL-UBE2O interaction.
(A) Stably-transfected, randomly integrated Halo-MLL HEK293 cells were stained with HaloTag R110 ligand and sorted by flow cytometry to get the low-expressing (Halo-MLLDim Halo-MLL cells.
(B) Representative sort for shGFP and TRC lentiviral libraries infected Halo-MLLDim cells. Halo-MLLDim cells were infected with lentiviral shRNA libraries or shGFP. Flow cytometry sorting was performed to obtain cells with increased Halo-MLL expression. Gating for cells with increased HaloTag R110 signal is indicated in pink.
(C) Identification of the IL-1 pathway in the shRNA screen. Components of the IL-1 pathway, including IL1R1, IL1RAP and TOLLIP were represented in the 303 enriched genes and are indicated with a red star (Table S2).
(D–F) Knockdown of IL-1 pathway components increases endogenous MLL protein. Knockdown of IL-1 pathway components and changes in MLL N320 protein levels were determined by western blotting. Fold changes of MLL N320 protein relative to shGFP are indicated. Tubulin serves as a loading control.
(G) IL-1β rapidly induces MLL N320 degradation in 293C6 cells, which have ectopically expressed IL-1 receptors IL1R and IL1RAP. 293C6 cells were stimulated with PBS or 50 ng/ml IL-1β for the indicated time. MG-132 was added prior to IL-1β induction. Fold changes relative to 0 minutes are indicated.
(H) IL-1β increases MLL-UBE2O interaction. Halo-MLL-Inter and Myc-UBE2O plasmids were cotransfected into 293C6 cells. 24 h later, these cells were stimulated with IL-1β for 30 min in the presence of MG-132 before MLL purification with HaloLink resin. Myc-UBE2O was detected with anti-Myc antibody and the inputs were blotted with anti-HaloTag and anti-Myc antibodies.
(I) UBE2O depletion disrupts IL-1β-induced MLL degradation. After UBE2O depletion for 4 days, 293C6 cells were stimulated with 50 ng/ml IL-1β for 90 min.
(J) IL-1β stimulates UBE2O–mediated MLL-Inter ubiquitination. His-tagged ubiquitin, Myc-UBE2O, and Halo-MLL-Inter plasmids were cotransfected into 293C6 as indicated. 24 h later, these cells were stimulated with IL-1β for 45 min in the presence of MG-132. His-tagged ubiquitinated proteins were purified with Ni-NTA agarose and blotted with anti-HaloTag antibody.
(K) IRAK4 directly phosphorylates UBE2O in vitro. 100 ng IRAK4 was incubated with eluates (purified from either vector or Flag-UBE2O transfected HEK293 cells) in the presence of γ-32P ATP. The phosphorylated IRAK4 and UBE2O proteins were visualized by autoradiography.
See also Figures S2 and Table S2.