Abstract
The management of small cell lung cancer (SCLC) has reached a plateau. Etoposide and platinum-based chemotherapy plus thoracic irradiation remain the standard treatment strategy for SCLC. Our study aims to assess the potential prognostic factors of patients treated with etoposide and platinum-based chemotherapy and explore which group of patients can benefit more from standard treatment strategies. On univariate analysis, age>65 years, male patients, KPS (Karnofsky Performance Status)≤80 points, positive smoking history, anemia, lymphocyte counts≤1.65×109/L, neutrophil to lymphocyte ratio (NLR)>3.18, lymphocyte to monocyte ratio (LMR)≤2.615, lactate dehydrogenase (LDH)>216.5 U/L, alkaline phosphatase (ALP)>119.5 U/L, absence of surgery, absence of thoracic irradiation, chemotherapy cycles<4, metastatic sites≥2 and extensive disease were correlated with a poor prognosis. Gender, KPS, chemotherapy cycles, thoracic irradiation, metastatic sites, LDH and tumor stage held statistical significance on multivariate analysis (p<0.05). High LDH was closely correlated with extensive disease, metastatic sites≥2, anemia, low LMR, high NLR and ALP levels. Subgroup analysis showed patients with male gender, KPS≤80 points, LDH≤216.5U/L, extensive disease and metastatic sites<2 could benefit more from ≥4 chemotherapy cycles. Patients with male gender, KPS>80 points, LDH≤216.5U/L, limited disease and metastatic sites<2 could benefit more from thoracic irradiation (p<0.05 on uni- and multivariate analysis). In conclusion, female patients, KPS>80 points, chemotherapy cycles≥4, thoracic irradiation, metastatic sites<2, LDH≤216.5U/L and limited disease were independent positive prognostic factors for SCLC patients treated with etoposide and platinum-based chemotherapy. Selected patients can benefit more from the management of ≥4 cycles of chemotherapy and thoracic irradiation.
Keywords: small cell lung cancer, prognostic factors, subgroup analysis, inflammation, survival
INTRODUCTION
SCLC is an aggressive disease with a high mortality and negative prognosis. Median survival is less than 1 year, with a 5-year survival of approximately 5% [1]. The management of SCLC has also reached a plateau for nearly 30 years. Etoposide and platinum-based chemotherapy plus thoracic irradiation remain the standard treatment strategy for SCLC [2]. However, treatment outcome is significantly different. Although patients received the same treatment strategies, many of them progressed quickly and even died soon. Systematic chemotherapy and thoracic radiotherapy have a limited role in these patients. On one hand, it leads to the increasing of economic burdens. On the other hand, it also adds some toxic reactions resulted from the treatment. The identification of patients who can benefit more from the treatment of chemotherapy and thoracic radiotherapy is of great value in clinics.
A number of retrospective studies have been performed to assess the prognostic roles of parameters in SCLC patients, but the patients enrolled are not treated with the same chemotherapy strategy and the results are not identical. Poor performance status, elevated serum LDH and extensive disease are usually associated with a short term survival in most studies [1, 3–5]. Alkaline phosphatase (ALP) [1, 5–8], age [9], gender [3, 8, 9], neutrophil [4, 9] and serum albumin [4, 6] were also found to be correlated with SCLC prognosis in some studies. The potential prognostic values of these parameters in SCLC patients treated with the same chemotherapy strategy remains unclear.
In clinics, we find the diagnosis of SCLC is usually accompanied by inflammation in most patients. Inflammation and the subsequent tumor development or tumor-elicited inflammation are critical steps for the development of cancers [10, 11]. Inflammation is often regarded as a feature of body innate immunity [11]. All types of immune cells can exist in the core, invasive margin of tumors as well as the adjacent tertiary lymphoid structures, and they can directly or indirectly affect tumor development through the secretion of cytokines [12–14]. Neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and lymphocyte to monocyte ratio (LMR), as key inflammatory markers, have been found to be associated with the prognosis of various malignancies including SCLC [15–21]. However, recent studies with small number of SCLC patients enrolled seldom assess the prognostic roles of some biochemical markers, lymphocyte, monocyte or LMR [16, 21]. And previous studies rarely involve the roles of lymphocyte, monocyte, NLR, PLR and LMR [1, 3–9]. We will take more serum biochemical markers into consideration to explore their prognostic values.
Our present study aims to assess the potential prognostic values of parameters in the presence of more serum biochemical markers in the whole group and variable subgroups of SCLC patients treated with the same chemotherapy strategy. But more importantly, we want to explore which group of patients can benefit more from the treatment of more cycles of etoposide and platinum-based chemotherapy and thoracic irradiation, and this is significant in clinics. It can help gain greater clinical benefits and survival time in the condition of smaller economic burdens and slighter toxic reactions.
RESULTS
Patient characteristics
This study included 707 patients (454 men, 253 women). The mean age was 56.24±10.15 years (range 23-75). Smoking history was present in 62.5% (442/707) patients (Table 1). The most common metastatic sites at diagnosis were bone (58/707, 8.2%), liver (47/707, 6.6%), brain (44/707, 6.2%) and adrenal glands (21/707, 3.0%). The most common symptoms at presentation were cough (597/707, 84.4%), shortness of breath (244/707, 34.5%) and chest pain (243/707, 34.4%). Of the 707 patients, 288 (40.7%) patients suffered ED, 419 (59.3%) patients suffered LD. All patients were treated with etoposide and platinum-based chemotherapy, and only 294 (41.6%) patients received thoracic radiotherapy. The KPS was ≤80 points in 164 cases (23.2%) and >80 points in 543 cases (76.8%). The median time from initial symptoms presented to definitive diagnosis was 1.0 months. The median survival time (MST) was 15.0 months (95% CI, 13.552-16.448) for all cases and 145 patients were still alive at present (Figure 1). Of all 145 cases, 109 had LD and 36 had ED. The survival rates at 1-, 2- and 5-year were 59.0%, 34.1% and 20.8%, respectively. The median survival time and 1-, 2-, 5-year survival rate for LD and ED were 21.0 months, 71.6%, 43.7%, 26.5% and 10.0 months, 40.6%, 20.1%, 12.5%, respectively.
Table 1. Clinical characteristics of patients with SCLC.
| Features | n | % |
|---|---|---|
| Age (years) | ||
| ≤65 | 561 | 79.3 |
| >65 | 146 | 20.7 |
| Gender | ||
| Male | 454 | 64.2 |
| Female | 253 | 35.8 |
| Symptoms initially presented | ||
| Cough | 597 | 84.4 |
| Shortness of breath | 244 | 34.5 |
| Chest pain | 243 | 34.4 |
| Hemoptysis or blood in phlegm | 233 | 33.0 |
| Hoarseness | 30 | 4.2 |
| KPS (Karnofsky Performance Status) | ||
| >80 point | 543 | 76.8 |
| ≤80 points | 164 | 23.2 |
| Smoking history | ||
| Yes | 442 | 62.5 |
| No | 265 | 37.5 |
| Family history of cancer | ||
| Yes | 116 | 16.4 |
| No | 591 | 83.6 |
| Thoracic irradiation | ||
| Yes | 294 | 41.6 |
| No | 413 | 58.4 |
| Surgery | ||
| Yes | 25 | 3.5 |
| No | 682 | 96.5 |
| Chemotherapy cycles | ||
| <4 | 332 | 47.0 |
| ≥4 | 375 | 53.0 |
| Metastatic sites | ||
| <2 | 642 | 90.8 |
| ≥2 | 65 | 9.2 |
| Stage | ||
| LD | 419 | 59.3 |
| ED | 288 | 40.7 |
Figure 1. Overall survival and independent prognostic factors for 707 patients treated with etoposide and platinum-based chemotherapy.
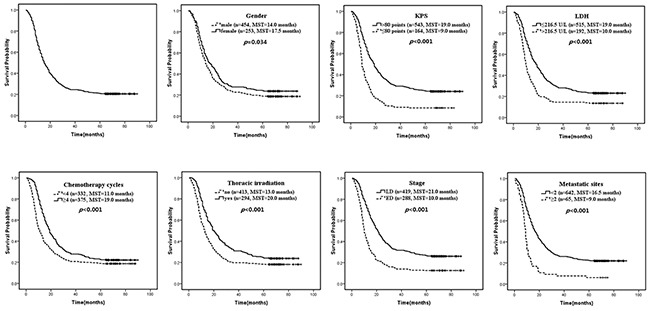
MST, median survival time.
The cutoff values for neutrophil, lymphocyte, monocyte, NLR, PLR, LMR, LDH and ALP
The mean (±SD) neutrophil, lymphocyte and monocyte counts, NLR, PLR, LMR, LDH and ALP were 4.55 (±2.01), 1.68 (±0.62), 0.49 (±0.23)×109/L, 3.16 (±2.44), 174.90 (±107.97), 5.72 (±15.43), 210.75 (±136.20) U/L and 104.47 (±52.89) U/L, respectively. The ROC analysis showed the optimal LDH cut-off was 216.5 U/L (p<0.001, AUC, 0.611; 95% CI, 0.569–0.652). The patients were then divided into high (>216.5 U/L) and low LDH (≤216.5 U/L) groups. There were 192 (27.2%) patients in the high group and 515 (72.8%) in the low LDH group. The ALP of 119.5 U/L (p=0.231, AUC, 0.526; 95% CI, 0.483–0.569), neutrophil of 4.95×109/L (p=0.577, AUC, 0.512; 95% CI, 0.469–0.555), lymphocyte of 1.65×109/L (p=0.009, AUC, 0.557; 95% CI, 0.514–0.599), monocyte of 0.495×109/L (p=0.183, AUC, 0.529; 95% CI, 0.486–0.571), NLR of 3.18 (p=0.037, AUC, 0.545; 95% CI, 0.503–0.588), PLR of 176.5 (p=0.194, AUC, 0.528; 95% CI, 0.486–0.571) and LMR of 2.615 (p=0.010, AUC, 0.556; 95% CI, 0.513–0.598) were selected as the optimal cut-off values.
Serum biochemical examinations
Routine blood test and liver function evaluation were performed on each patient. The most common abnormalities were high levels of LMR (512/707,72.4%), lymphocyte (334/707, 47.2%), monocyte (321/707, 45.4%), ALT (268/707, 37.9%), neutrophil (248/707, 35.1%), LDH (192/707, 27.2%), platelet (160/707, 22.6%), low albumin level (105/707, 14.9%) and anemia (64/707, 9.1%). Carcinoembryonic antigen (CEA) and neuron specific enolase (NSE) were tested in 402 and 344 patients, and 29.1% (117/402) and 86.9% (299/344) patients showed an elevated level of CEA (>5 ng/ml) and NSE (>15.2 ng/ml), respectively.
Univariate analysis
Each parameter was assessed by univariate analysis. Significant prognostic factors were age (p=0.025), gender (p=0.034), KPS (p<0.001), smoking history (p=0.009), anemia (p=0.026), lymphocyte counts (p=0.028), NLR (p=0.002), LMR (p=0.008), LDH (p<0.001), ALP (p=0.015), surgery (p=0.011), thoracic irradiation (p<0.001), chemotherapy cycles (p<0.001), metastatic sites (p<0.001) and tumor stage (p<0.001). We also assessed the prognostic values of CEA (n=402) and NSE (n=344). High NSE level was a negative prognostic factor on univariate analysis (>15.2 vs ≤15.2 ng/ml, 14.0 vs 24.0 months, p=0.009). No significant difference was found on variable CEA levels (>5 vs ≤5 ng/ml, 13.0 vs 15.5 months, p=0.219). These data showed the following factors were correlated with a poor prognosis: age>65 years, male patients, KPS≤80 points, positive smoking history, anemia (a hemoglobin value<12.0 g/dl in men and 11.0 g/dl in women), lymphocyte counts<1.65×109/L, NLR>3.18, LMR≤2.615, LDH>216.5 U/L, ALP>119.5 U/L, NSE>15.2 ng/ml, absence of surgery, absence of thoracic irradiation, chemotherapy cycles<4, metastatic sites≥2 and extensive disease (Table 2). The variables examined in the final multivariate analysis were age, gender, KPS, smoking history, anemia, lymphocyte counts, NLR, LMR, LDH, ALP, surgery, thoracic irradiation, chemotherapy cycles, metastatic sites and tumor stage.
Table 2. Univariate analysis results of potential prognostic factors of SCLC patients treated with etoposide and platinum-based chemotherapy.
| Variables | O/N* | % | median survival time (months) | 95% CI | p value |
|---|---|---|---|---|---|
| Age (years) | 0.025 | ||||
| ≤65 | 436/561 | 77.7 | 16.0 | 14.129-17.871 | |
| >65 | 126/146 | 86.3 | 15.0 | 12.787-17.213 | |
| Gender | 0.034 | ||||
| Male | 369/454 | 81.3 | 14.0 | 12.394-15.606 | |
| Female | 193/253 | 76.3 | 17.5 | 14.090-20.910 | |
| KPS | <0.001 | ||||
| >80 point | 412/543 | 75.9 | 19.0 | 16.943-21.057 | |
| ≤80 points | 150/164 | 91.5 | 9.0 | 8.105-9.895 | |
| Smoking history | 0.009 | ||||
| Yes | 364/442 | 82.4 | 14.0 | 12.640-15.360 | |
| No | 198/265 | 74.7 | 18.5 | 15.367-21.633 | |
| Family history of cancer | 0.180 | ||||
| Yes | 98/116 | 84.5 | 13.0 | 9.047-16.953 | |
| No | 464/591 | 78.5 | 15.5 | 14.011-16.989 | |
| Anemia | 0.026 | ||||
| Yes | 55/64 | 85.9 | 11.5 | 8.070-14.930 | |
| No | 507/643 | 78.8 | 16.0 | 14.469-17.531 | |
| RBC | 0.184 | ||||
| normal | 500/629 | 79.5 | 16.0 | 14.485-17.515 | |
| Below normal | 62/78 | 79.5 | 10.0 | 6.634-13.366 | |
| WBC (×109/L) | 0.135 | ||||
| ≤10 | 522/652 | 80.1 | 15.0 | 13.344-16.656 | |
| >10 | 40/55 | 72.7 | 19.0 | 9.929-28.071 | |
| Platelet | 0.662 | ||||
| ≤300 | 438/547 | 80.1 | 15.0 | 13.472-16.528 | |
| >300 | 124/160 | 77.5 | 14.5 | 11.143-17.857 | |
| Neutrophil (×109/L) | 0.518 | ||||
| ≤4.95 | 364/495 | 79.3 | 16.0 | 14.125-17.875 | |
| >4.95 | 198/248 | 79.8 | 14.0 | 12.404-15.596 | |
| Lymphocyte (×109/L) | 0.028 | ||||
| ≤1.65 | 303/373 | 81.2 | 13.0 | 11.675-14.325 | |
| >1.65 | 259/334 | 77.5 | 18.0 | 15.442-20.558 | |
| Monocyte (×109/L) | 0.117 | ||||
| ≤0.495 | 303/386 | 78.5 | 17.5 | 15.531-19.469 | |
| >0.495 | 259/321 | 80.7 | 14.0 | 12.465-15.535 | |
| NLR | 0.002 | ||||
| ≤3.18 | 357/462 | 77.3 | 17.0 | 14.894-19.106 | |
| >3.18 | 205/245 | 83.7 | 13.0 | 11.014-14.986 | |
| PLR | 0.092 | ||||
| ≤176.5 | 350/445 | 78.7 | 17.0 | 15.211-18.789 | |
| >176.5 | 212/262 | 80.9 | 13.0 | 11.601-14.399 | |
| LMR | 0.008 | ||||
| ≤2.615 | 161/195 | 82.6 | 12.0 | 10.422-13.578 | |
| >2.615 | 401/512 | 78.3 | 17.0 | 15.152-18.848 | |
| AST (U/L) | 0.194 | ||||
| ≤40 | 495/623 | 79.5 | 16.0 | 14.589-17.411 | |
| >40 | 67/84 | 79.8 | 9.5 | 7.404-11.596 | |
| ALT (U/L) | 0.574 | ||||
| ≤40 | 351/439 | 80.0 | 16.5 | 14.492-18.508 | |
| >40 | 211/268 | 78.7 | 14.0 | 12.102-15.898 | |
| LDH (U/L) | <0.001 | ||||
| ≤216.5 | 396/515 | 76.9 | 19.0 | 16.737-21.263 | |
| >216.5 | 166/192 | 86.4 | 10.0 | 8.869-11.131 | |
| ALP (U/L) | 0.015 | ||||
| ≤119.5 | 431/547 | 78.8 | 16.5 | 14.737-18.263 | |
| >119.5 | 131/160 | 81.9 | 12.0 | 9.717-14.283 | |
| Serum albumin (g/L) | 0.378 | ||||
| ≤40 | 85/105 | 81.0 | 14.0 | 9.816-18.184 | |
| >40 | 477/602 | 79.2 | 15.5 | 14.018-16.982 | |
| Globulin (G, g/L) | 0.123 | ||||
| ≤35 | 522/651 | 80.2 | 15.0 | 13.626-16.374 | |
| >35 | 40/56 | 71.4 | 20.0 | 12.055-27.945 | |
| Surgery | 0.011 | ||||
| Yes | 15/25 | 60.0 | 31.0 | 0.000-69.353 | |
| No | 547/682 | 80.2 | 15.0 | 13.707-16.293 | |
| Thoracic irradiation | <0.001 | ||||
| Yes | 224/294 | 76.2 | 20.0 | 16.849-23.151 | |
| No | 338/413 | 81.8 | 13.0 | 11.662-14.338 | |
| Chemotherapy cycles | <0.001 | ||||
| <4 | 270/332 | 81.3 | 11.0 | 9.215-12.785 | |
| ≤4 | 292/375 | 77.9 | 19.0 | 16.919-21.081 | |
| Metastatic sites | <0.001 | ||||
| <2 | 501/642 | 78.0 | 16.5 | 14.969-18.031 | |
| ≤2 | 61/65 | 93.8 | 9.0 | 8.022-9.978 | |
| Stage | <0.001 | ||||
| LD | 310/419 | 74.0 | 21.0 | 18.668-23.332 | |
| ED | 252/288 | 87.5 | 10.0 | 9.050-10.950 |
O/N=observed death number/total patient number in each group
Multivariate analysis
The multivariate analysis showed gender (p=0.027), KPS (p<0.001), LDH (p=0.001), chemotherapy cycles (p=0.005), thoracic irradiation (p=0.013), metastatic sites (p=0.015) and tumor stage (p<0.001) were statistically significant (Table 3). The hazard ratios of death were observed for LDH≥216.5U/L (1.415 fold increase compared to LDH<216.5 U/L), KPS≤80 points (1.834 fold increase compared to KPS>80 points), chemotherapy cycles<4 (1.276 fold increase compared to chemotherapy cycles≥4), absence of thoracic irradiation (1.25 fold compared to the presence of thoracic irradiation), extensive disease (1.428 fold compared to limited disease). Female patients, KPS>80 points, LDH≤216.5U/L, chemotherapy cycles≥4, thoracic irradiation, metastatic sites<2 and limited disease were independent positive prognostic factors for long-term survival of SCLC patients treated with etoposide and platinum-based chemotherapy.
Table 3. Multivariate analysis of risk factors for SCLC patients treated with etoposide and platinum-based chemotherapy.
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Gender | 0.809 | 0.670-0.976 | 0.027 |
| Age | 0.985 | 0.802-1.210 | 0.888 |
| KPS | 1.834 | 1.498-2.245 | <0.001 |
| Smoking history | 1.148 | 0.954-1.382 | 0.145 |
| Anemia | 1.060 | 0.794-1.416 | 0.691 |
| Lymphocyte | 0.879 | 0.729-1.059 | 0.176 |
| NLR | 1.030 | 0.837-1.267 | 0.780 |
| LMR | 1.053 | 0.848-1.307 | 0.641 |
| LDH | 1.415 | 1.161-1.724 | 0.001 |
| ALP | 1.002 | 0.818-1.228 | 0.984 |
| Surgery | 0.725 | 0.429-1.226 | 0.231 |
| Chemotherapy cycles | 0.784 | 0.660-0.931 | 0.005 |
| Thoracic irradiation | 0.800 | 0.671-0.954 | 0.013 |
| Metastatic sites | 1.440 | 1.073-1.934 | 0.015 |
| Stage | 1.428 | 1.177-1.732 | <0.001 |
The association between LDH and clinicopathological characteristics
LDH was identified to be serum independent prognostic factor of the whole SCLC patients on uni- and multivariate analysis. The optimal cutoff points to assess the difference of overall survival were 216.5 U/L for LDH. Using the optimal cutoff points, we divided all patients into two groups to evaluate the association of LDH levels and clinicopathological characteristics of SCLC patients (Table 4). We found that high levels of LDH was correlated with the presence of anemia (yes vs no, 39.1% vs 26.0%, p=0.025) and high NLR (yes vs no, 35.1% vs 22.9%, p=0.001), low LMR (yes vs no, 42.1% vs 21.5%, p<0.001), high ALP level (yes vs no, 36.2% vs 24.5%, p<0.001), extensive disease (yes vs no, 42.0% vs 16.9%, p<0.001) and more metastatic sites at diagnosis (yes vs no, 55.4% vs 24.3%, p<0.001). High LDH could be more frequently seen in patients with extensive disease, metastatic sites≥2, anemia, low LMR and high NLR levels.
Table 4. Correlation of the variable LDH level with the clinicopathological characteristics of SCLC patients.
| Variable | LDH (U/L) | ||
|---|---|---|---|
| ≤216.5n (%) | >216.5n (%) | p value | |
| Age (years) | 0.051 | ||
| ≤65 | 418(74.5) | 143(25.5) | |
| >65 | 97(66.4) | 49(33.6) | |
| Gender | 0.449 | ||
| Male | 335(73.8) | 119(26.2) | |
| Female | 180(71.1) | 73(28.9) | |
| KPS | 0.274 | ||
| >80 points | 401(73.8) | 142(26.2) | |
| ≤80 points | 114(69.5) | 50(30.5) | |
| Smoking history | 0.224 | ||
| Yes | 315(71.3) | 127(28.7) | |
| No | 200(75.5) | 65(24.5) | |
| Anemia | 0.025 | ||
| Yes | 39(60.9) | 25(39.1) | |
| No | 476(74.0) | 167(26.0) | |
| Lymphocyte(×109/L) | 0.426 | ||
| ≤1.65 | 267(71.6) | 106(28.4) | |
| >1.65 | 248(74.3) | 86(25.7) | |
| NLR | 0.001 | ||
| ≤3.18 | 356(77.1) | 106(22.9) | |
| >3.18 | 159(64.9) | 86(35.1) | |
| LMR | <0.001 | ||
| ≤2.615 | 113(57.9) | 82(42.1) | |
| >2.615 | 402(78.5) | 110(21.5) | |
| ALP (U/L) | 0.003 | ||
| ≤119.5 | 413(75.5) | 134(24.5) | |
| >119.5 | 102(63.8) | 58(36.2) | |
| Stage | <0.001 | ||
| LD | 348(83.1) | 71(16.9) | |
| ED | 167(58.0) | 121(42.0) | |
| Metastatic sites | <0.001 | ||
| <2 | 486(75.7) | 156(24.3) | |
| ≥2 | 29(44.6) | 36(55.4) | |
Predictive prognosis analysis for variable subgroups in patients with SCLC
Subgroup analysis were performed to assess parameters affecting the prognosis of patients in different groups and identify which group of patients can benefit more from the treatment of chemotherapy cycles≥4 and thoracic radiotherapy. Subgroup analysis was performed in variable KPS, LDH, gender, tumor stage and metastatic sites groups based on multivariate analysis results. The parameters were the same with Table 2. Tumor stage, thoracic irradiation, metastatic sites and LDH were independent prognostic factors of KPS>80 points patients (p<0.05 on uni- and multivariate analysis). Tumor stage and chemotherapy cycles were independent prognostic factors in patients with KPS≤80 points. Details of independent prognostic factors in other subgroups analysis can be seen in Table 5.
Table 5. Suggested prognostic factors in variable subgroups of SCLC patients.
| Variables | KPS (points) | LDH (U/L) | tumor stage | metastatic sites | Gender | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| >80 | ≤80 | ≤216.5 | >216.5 | LD | ED | <2 | ≥2 | male | female | |
| Surgery | +/− | −/Ø | +/− | −/Ø | −/Ø | −/Ø | +/− | Ø/Ø | −/Ø | +/− |
| Stage | +/+ | +/+ | +/+ | +/+ | Ø/Ø | Ø/Ø | +/+ | Ø/Ø | +/+ | +/+ |
| Chemotherapy cycles | +/− | +/+ | +/+ | −/Ø | −/Ø | +/+ | +/+ | −/Ø | +/+ | +/− |
| Thoracic irradiation | +/+ | +/− | +/+ | +/− | +/+ | +/− | +/+ | −/Ø | +/+ | +/− |
| Metastatic sites | +/+ | −/Ø | +/− | +/− | Ø/Ø | +/+ | Ø/Ø | Ø/Ø | +/+ | +/− |
| Smoking history | +/− | −/Ø | −/Ø | +/+ | +/+ | −/Ø | +/− | −/Ø | −/Ø | +/− |
| NLR | +/− | −/Ø | +/− | −/Ø | −/Ø | +/− | +/− | −/Ø | +/− | −/Ø |
| LMR | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | +/− | −/Ø | −/Ø | +/− | −/Ø |
| Gender | +/− | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | +/− | −/Ø | −/Ø | −/Ø |
| Anemia | +/− | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | +/− | −/Ø | −/Ø | −/Ø |
| RBC | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | +/− |
| LDH | +/+ | +/− | Ø/Ø | Ø/Ø | +/+ | +/+ | +/+ | +/+ | +/+ | +/− |
| ALP | +/− | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | +/− | −/Ø | −/Ø | −/Ø |
| Age | −/Ø | −/Ø | +/− | −/Ø | +/− | −/Ø | +/− | −/Ø | −/Ø | −/Ø |
| PS | Ø/Ø | Ø/Ø | +/+ | +/+ | +/+ | +/+ | +/+ | −/Ø | +/+ | +/+ |
| WBC | −/Ø | −/Ø | −/Ø | +/+ | −/Ø | −/Ø | −/Ø | +/+ | −/Ø | −/Ø |
| Family history | −/Ø | −/Ø | −/Ø | −/Ø | +/+ | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø |
| Lymphocytes | −/Ø | −/Ø | −/Ø | +/− | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø |
| Neutrophils | −/Ø | −/Ø | −/Ø | +/+ | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø | −/Ø |
+/+=significant on both univariate and multivariate analysis; +/−=significant on univariate analysis, not significant on multivariate analysis; −/Ø=not significant on univariate analysis and not assessed on multivariate analysis; Ø/Ø= not studied, the parameters used were the same with Table 2 and factors with no statistical significance in variable subgroups were not shown.
Subgroup analysis showed that patients with male gender, KPS≤80 points, LDH≤216.5U/L, extensive disease and metastatic sites<2 can benefit more from ≥4 chemotherapy cycles (p<0.05 on uni- and multivariate analysis, Figure 2). In addition, patients with male gender, KPS>80 points, LDH≤216.5U/L, limited disease and metastatic sites<2 can benefit more from thoracic irradiation (Figure 3). Patients with PS>80 points and female gender can get survival benefits from more cycles of chemotherapy on univariate analysis (p<0.05), but it holds no significance on multivariate analysis. Patients with LDH>216.5U/L, limited disease and metastatic sites≥2 can get little benefits from more cycles of chemotherapy (p>0.05). Patients with KPS≤80 points, LDH>216.5 U/L, female gender and extensive disease can benefit from thoracic irradiation on univariate analysis (p<0.05), but it holds no significant difference on multivariate analysis. Patients with metastatic sites≥2 can get little benefit from the treatment of chemotherapy cycles≥4 and thoracic irradiation (p>0.05).
Figure 2. Patients with male gender, KPS≤80 points, LDH≤216.

5U/L, extensive disease and metastatic sites<2 can benefit more from the management of etoposide and platinum-based chemotherapy (p<0.05 on uni- and multivariate analysis). MST, median survival time.
Figure 3. Patients with male gender, KPS>80 points, LDH≤216.

5U/L, limited disease and metastatic sites<2 can benefit more from the treatment of thoracic irradiation (p<0.05 on uni- and multivariate analysis). MST, median survival time.
DISCUSSION
In our study, we find that female patients, KPS>80 points, LDH≤216.5U/L, chemotherapy cycles≥4, thoracic irradiation, metastatic sites<2 and limited disease are independent positive prognostic factors for SCLC treated with etoposide and platinum-based chemotherapy. Selected patients can benefit more from the treatment of chemotherapy cycles≥4 and thoracic radiotherapy. Patients with male gender, KPS≤80 points, LDH≤216.5U/L, extensive disease and metastatic sites<2 can benefit more from ≥4cycles of chemotherapy. And patients with male gender, KPS>80 points, LDH≤216.5U/L, limited disease and metastatic sites<2 can benefit more from thoracic radiotherapy. Inflammatory markers of NLR and LMR are associated with the prognosis of SCLC patients, but it holds no statistical significance on multivariate and subgroup analysis. Serum LDH, which can be easily available, still has an independent prognostic values in the condition of more parameters and these results are valuable in clinics.
SCLC is a fatal disease characterized as early recurrence and rapid progression. Many patients progressed quickly and died soon even they received the same treatment strategies. So the identification of patients who can benefit more from the same treatment strategy is important. LDH<216.5 U/L and metastatic sites<2 reflect a low tumor burdens. Subgroup analysis shows chemotherapy cycles≥4 has more significant role in patients with tolerated performance status, male gender, low tumor burdens and relatively extensive disease. Thoracic radiotherapy is more effective in the treatment of patients with better tolerance, limited features, male gender and low tumor burdens. Patients with LDH<216.5 U/L, male gender and metastatic sites<2 can significantly benefit from ≥cycles of chemotherapy and thoracic irradiation. The overall survival benefits of patients with metastatic sites≥2 got from these two treatment is limited, and the treatment in these cases should be carefully considered. Female gender can benefit from these two treatment strategies, but no statistical significance is found on multivariate analysis. Maybe the sample size and grouping of bias can partly account for this result, and the roles of these treatment in female gender still need to be verified. These results can help us identify which treatment strategy is more suitable for variable groups of patients. In addition, it can avoid some additional toxic reactions for patients who can benefit little from ≥4cycles of chemotherapy and thoracic irradiation. This is important for a long-term survival and better quality of life.
Inflammation plays an important role in cancer development, and inflammatory markers of NLR, PLR, LMR et al have been demonstrated to be associated with the prognosis of various malignancies [10, 11, 15–21]. White blood cell (WBC) counts can reflect body inflammatory status, and it plays limited role in the prognosis of the whole SCLC patients (p>0.05). However, subgroup analysis shows WBC counts>10×109/L is a positive independent prognostic factor for patients with LDH>216.5 U/L and metastatic sites≥2. WBC may have an important role in patients with high tumor burdens and advanced stage. Similar phenomenon can also be found in previous study and the mechanism remains unknown [1]. High NLR and low LMR are associated with the prognosis of SCLC patients with the presence of more parameters, but no statistical significance is found on multivariate analysis. High NLR reflects a status of neutrophilia or lymphocytopenia. Vascular endothelial growth factor (VEGF), as a proangiogenic factor, is mainly secreted by neutrophils and it plays a critical role in cancer progression [22]. Lymphocytopenia indicates a poor body lymphocyte mediated immune status to cancer. Recent study reveals high NLR is correlated with a distinct cytokine profile related to key biological processes involved in carcinogenesis including an increased expression of interleukin 6 (IL-6), IL-8 and VEGF et al [23]. High NLR and low LMR can promote tumor angiogenesis and inhibit body immunity, leading to a poor prognosis. The potential roles of these inflammatory markers still need to be verified in further research.
LDH has been identified as prognostic factor of SCLC and various malignancies in previous studies [1,5–8,16,21,24–29]. However, the mechanism remains poorly understood. In our study, we found, taking into account of more parameters, LDH still holds statistical significance on a series of analysis. High LDH level is a negative independent prognostic factors of SCLC patients. LDH plays an important role in energy production in various cell types, and an elevated LDH level may promote tumor progression by regulating tumor metabolism [29]. High LDH also reflects a higher tumor burden, the extent of disease and rapid turnover of tumor cells [5,29]. In our study, high LDH is also found to be associated with high ALP level, and patients with elevated ALP show worse treatment tolerance and response in the analysis of overall survival and progression free survival (PFS) [26,29]. In addition, elevated ALP is also correlated with a high tumor burden and can promote tumor progression [29]. Patients with LDH≤216.5 U/L can significantly benefit from the treatment of ≥4 cycles of chemotherapy and thoracic irradiation on subgroup analysis. These results may have some hints for our clinical decisions for the treatment of patients with variable LDH levels.
There are some limitations of our study. First, it is a single constitutional retrospective analysis and we exclude many patients treated with best supportive care or other treatment strategies in order to assess the prognostic roles of parameters in patients treated with etoposide and platinum-based chemotherapy. That is why there are more limited disease patients in our cohort. Second, we do not assess the impact of these factors on PFS for the absence of PFS information in some patients. In addition, patients number in some groups is relatively small. We will pay more attention to these details in our further study.
In conclusion, female patients, KPS>80 points, LDH≤216.5U/L, chemotherapy cycles≥4, thoracic irradiation, metastatic sites<2 and limited disease are independent positive prognostic factors for patients treated with etoposide and platinum-based chemotherapy. Patients with male gender, KPS≤80 points, LDH≤216.5U/L, extensive disease and metastatic sites<2 can benefit more from ≥4cycles of chemotherapy. And patients with male gender, KPS>80 points, LDH≤216.5U/L, limited disease and metastatic sites<2 can benefit more from thoracic radiotherapy. Further retrospective and prospective studies are urgently needed to provide evidence-based recommendations for our clinics.
MATERIALS AND METHODS
Patients
We conducted a retrospective analysis on 1126 cases with pathologically confirmed SCLC who were registered and followed up in our hospital between January 2008 and January 2010. Only patients treated with etoposide and platinum-based chemotherapy were included in this study. The criteria for entry into our study were as follows: 1. histologically or cytologically proved SCLC; 2. no prior anti-tumor therapies; 3. age≤75 years; 4. complete follow-up information; 5. combined routine blood test and liver function examinations. Finally, 707 patients were included in our study. Our study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital and written informed consent was obtained from each patient. Limited disease (LD) is defined as disease confined to the ipsilateral chest within a single radiation field, while extensive disease (ED) was defined as disease beyond the ipsilateral hemithorax including malignant pleural, pericardial effusion, or hematogenous metastasis.
Data collection
The following parameters were collected and evaluated for prognostic impact: age (≤65 or >65 years), gender, KPS (Karnofsky Performance Status), smoking history, family history of cancer, serum red blood cell (RBC), platelet (≤300,000 or >300,000/μl), white blood cell (WBC, ≤10 or >10×109/L), hemoglobin, neutrophil (≤4.95 or >4.95×109/L), lymphocyte (≤1.65 or >1.65×109/L), monocyte (≤0.495 or >0.495×109/L), NLR (≤3.18 or >3.18), PLR (≤176.5 or >176.5), LMR (≤2.615 or >2.615), aspartate aminotransferase (AST, ≤40 or >40 U/L), alanine aminotransferase (ALT, ≤40 or >40 U/L), LDH (≤216.5 or >216.5 U/L), ALP (≤119.5 or >119.5 U/L), albumin (≤40 or >40 g/L), globulin (≤35 or >35 g/L), surgery, chemotherapy cycles, thoracic irradiation, metastatic sites and tumor stage at initial diagnosis. A low serum RBC level was defined as a red blood cell count <3.5 or 4.0×109/L in females and males, respectively. Anemia is defined as a hemoglobin value<12.0 g/dl in men and 11.0 g/dl in women. The NLR, PLR, and LMR values were calculated using the neutrophil, platelet, lymphocyte and monocyte counts as the ratio of neutrophil and platelet counts to lymphocyte or lymphocyte counts to monocyte, respectively.
Statistical analysis
Mean values±standard deviation (SD) or median and range were calculated for continuous variables. The optimal cut-off point for neutrophil, lymphocyte, monocyte, NLR, PLR, LMR, LDH and ALP were calculated by a receiver operating characteristic (ROC) curve analysis. Survival outcomes were dichotomized by survival status (alive or dead) until a defined time of the median survival time of 15.0 months in the ROC curve analysis. The overall survival time was defined from diagnosis to death or the last follow-up of April 2015 if the patients were still alive. The survival curves and 95% confidence intervals (CIs) were obtained using the Kaplan-Meier method, and the survival was compared by log-rank test. The relationship between LDH and the clinicopathological features were evaluated using the chi-squared test. Univariate analysis was used to examine the potential prognostic roles of all factors. The prognostic factors with p-value≤0.05 in the univariate analysis were examined in the multivariate analysis. SPSS 16.0 statistical software was used for statistical analysis. All P-values≤0.05 were considered statistically significant.
Acknowledgments
This work was supported by National Natural Scientific Foundation of China (No: 81572824, 81673007 and 81672931), Health Department of Heilongjiang Provincial of China (No: 2011-124), Harbin Medical University Cancer Hosptial major project Foundation (No: JJZ-2010-01), Provincial Department of Education (No: 12521284).
Footnotes
CONFLICTS OF INTEREST
None declared.
REFERENCES
- 1.Kawahara M, Fukuoka M, Saijo N, Nishiwaki Y, Ikegami H, Tamura T, Shimoyama M, Suemasu K, Furuse K. Prognostic factors and prognostic staging system for small cell lung cancer. Jpn J Clin Oncol. 1997(27):158–65. doi: 10.1093/jjco/27.3.158. [DOI] [PubMed] [Google Scholar]
- 2.Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007(12):1096–104. doi: 10.1634/theoncologist.12-9-1096. [DOI] [PubMed] [Google Scholar]
- 3.Osterlind K, Andersen PK. Prognostic factors in small cell lung cancer: multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res. 1986(46):4189–94. [PubMed] [Google Scholar]
- 4.Maestu I, Pastor M, Gómez-Codina J, Aparicio J, Oltra A, Herranz C, Montalar J, Munárriz B, Reynés G. Pretreatment prognostic factors for survival in small-cell lung cancer: a new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol. 1997(8):547–53. doi: 10.1023/a:1008212826956. [DOI] [PubMed] [Google Scholar]
- 5.Cerny T, Blair V, Anderson H, Bramwell V, Thatcher N. Pretreatment prognostic factors and scoring system in 407 small-cell lung cancer patients. Int J Cancer. 1987(39):146–9. doi: 10.1002/ijc.2910390204. [DOI] [PubMed] [Google Scholar]
- 6.Souhami RL, Bradbury I, Geddes DM, Spiro SG, Harper PG, Tobias JS. Prognostic significance of laboratory parameters measured at diagnosis in small cell carcinoma of the lung. Cancer Res. 1985(45):2878–82. [PubMed] [Google Scholar]
- 7.Rawson NS, Peto J. An overview of prognostic factors in small cell lung cancer. A report from the subcommittee for the management of lung cancer of the United Kingdom coordinating committee on cancer research. Br J Cancer. 1990(61):597–604. doi: 10.1038/bjc.1990.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dearing MP, Steinberg SM, Phelps R, Anderson MJ, Mulshine JL, Ihde DC, Johnson BE. Outcome of patients with small-cell lung cancer: effect of changes in staging procedures and imaging technology on prognostic factors over 14 years. J Clin Oncol. 1990(8):1042–9. doi: 10.1200/JCO.1990.8.6.1042. [DOI] [PubMed] [Google Scholar]
- 9.Paesmans M, Sculier JP, Lecomte J, Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Cutsem O, Mommen P, Ninane V, Klastersky J. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4consecutive prospective trials with a minimum follow-up of 5 years. Cancer. 2000(89):523–33. doi: 10.1002/1097-0142(20000801)89:3<523::aid-cncr7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008(454):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015(125):3347–55. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012(12):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 13.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013(31):860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 14.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011(71):5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Yao Y, Bai C, Zeng J, Shi D, Gu X, Shi X, Song Y. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac Cancer. 2015(6):275–87. doi: 10.1111/1759-7714.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go SI, Kim RB, Song HN, Kang MH, Lee US, Choi HJ, Lee SJ, Cho YJ, Jeong YY, Kim HC, Lee JD, Kim SH, Kang JH, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol. 2014(31):323. doi: 10.1007/s12032-014-0323-y. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi T, Fujimoto N, Nishi H, Miyamoto Y, Hara N, Asano M, Fuchimoto Y, Wada S, Kitamura K, Ozaki S, Kishimoto T. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer. 2015(90):111–7. doi: 10.1016/j.lungcan.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhang GM, Zhu Y, Luo L, Wan FN, Zhu YP, Sun LJ, Ye DW. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol. 2015(36):8537–43. doi: 10.1007/s13277-015-3613-x. [DOI] [PubMed] [Google Scholar]
- 19.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014(31):305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 20.Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen K, Su F. The Peripheral Blood Neutrophil-To Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple Negative Breast Cancer Patients. PLoS One. 2015(10):e0143061. doi: 10.1371/journal.pone.0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Jiang R, Li K. Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell Biochem Biophys. 2014(69):633–40. doi: 10.1007/s12013-014-9845-3. [DOI] [PubMed] [Google Scholar]
- 22.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003(6):283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z-Y, Raghav K, Lieu C H, Jiang Z-Q, Eng C, Vauthey J-N, Chang G J, Qiao W, Morris J, Hong D, Hoff P, Tran H, Menter D G, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088–97. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer-A retrospective single institution analysis. Respir Med. 2010(104):1937–42. doi: 10.1016/j.rmed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Weide B, Elsässer M, Büttner P, Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Witte M, Meier F, Garbe C. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer. 2012(107):422–8. doi: 10.1038/bjc.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maisano R, Azzarello D, P Del Medico, Maisano M, Bottari M, Egitto G, Nardi M. Alkaline phosphatase levels as a prognostic factor in metastatic colorectal cancer treated with the FOLFOX 4 regimen: a monoinstitutional retrospective study. Tumori. 2011(97):39–42. doi: 10.1177/030089161109700108. [DOI] [PubMed] [Google Scholar]
- 27.Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, Eisenberger MA, Tannock IF. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012(30):607–13. doi: 10.1016/j.urolonc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Neofytou K, Giakoustidis A, Mudan S. CALy Prognostic Score (CEA ALP Lymphocyte Count) as a predictor of survival after hepatectomy for liver-only colorectal metastases. J BUON. 2015(20):746–55. [PubMed] [Google Scholar]
- 29.Wei XL, Zhang DS, He MM, Jin Y, Wang DS, Zhou YX, Bai L, Li ZZ, Luo HY, Wang FH, Xu RH. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol. 2016(37):1879–87. doi: 10.1007/s13277-015-3851-y. [DOI] [PubMed] [Google Scholar]


