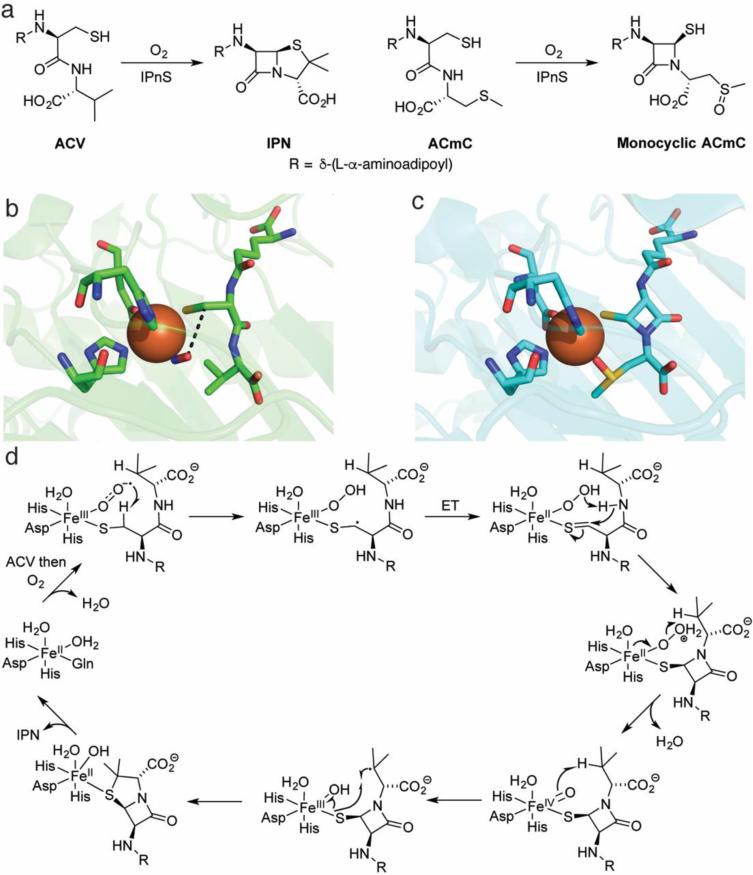
Fig. 2.
Overview of the chemistry effected by IPNS. a The native substrate of IPNS catalysis (left) and a substrate analog that does not undergo a second cyclization (right). b The crystal structure of the ternary complex of Fe(II)-IPNS-ACV-NO (PDB ID: 1BLZ). The distance between the oxygen atom of NO to the β carbon of cysteine in ACV is 3.3 Å (black dashes). c The structure of the product of the in crystallo reaction of Fe(II)-IPNS with ACmC reveals formation of the thiazolidine ring and sulfoxidation of the methylcysteine moiety (PDB ID: 1QJF). d A proposed mechanism for catalysis by IPNS. Instead of a formal ET in the second step after substrate binding, the two structures could also be considered resonance forms. For details, see the text