Abstract
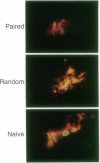
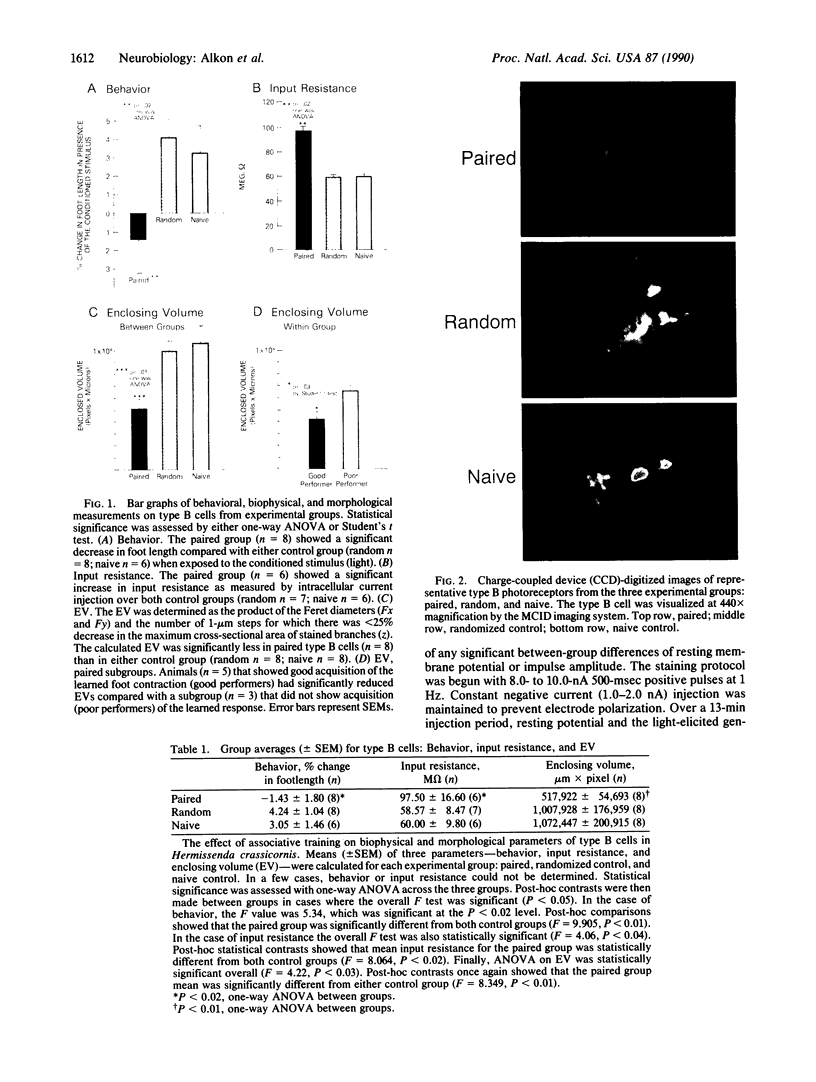
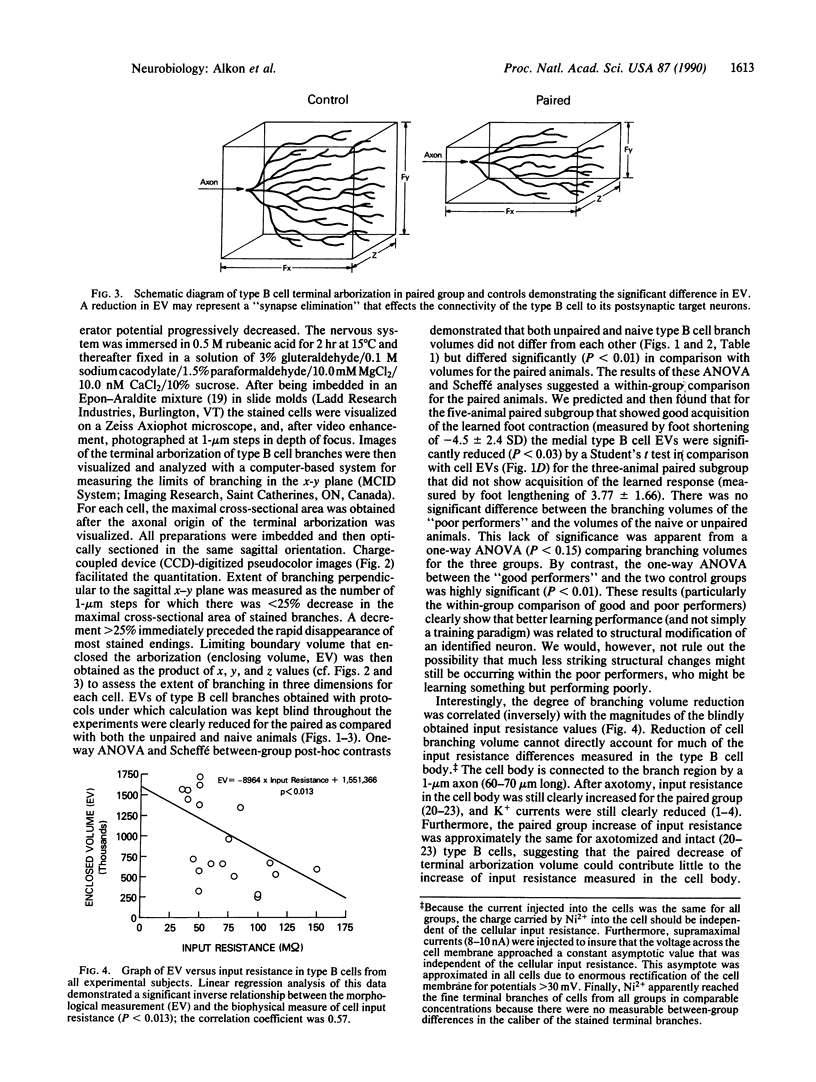
Associative memory of the mollusc Hermissenda crassicornis, previously correlated with changes of specific K+ currents, protein phosphorylation, and increased synthesis of mRNA and specific proteins, is here shown to be accompanied by macroscopic alteration in the structure of a single identified neuron, the medial type B photoreceptor cell. Four to five days after training, terminal arborizations of B cells iontophoretically injected with Ni2+ ions and then treated with rubeanic acid were measured with charge-coupled device (CCD)-digitized pseudocolor images of optical sections under "blind" conditions. Boundary volumes enclosing medial-type B-cell arborizations from classically conditioned animals were unequivocally reduced compared with volumes for naive animals or those trained with unpaired stimuli. Branch volume magnitude was correlated with input resistance of the medial type B-cell soma. Such associative learning-induced structural changes may share function with "synapse elimination" described in developmental contexts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Lederhendler I., Shoukimas J. J. Primary changes of membrane currents during retention of associative learning. Science. 1982 Feb 5;215(4533):693–695. doi: 10.1126/science.7058334. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Sakakibara M., Forman R., Harrigan J., Lederhendler I., Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol. 1985 Sep;44(2):278–300. doi: 10.1016/s0163-1047(85)90296-1. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Ikeno H., Harrigan J. F., Lederhendler I., Alkon D. L. Sequential modification of membrane currents with classical conditioning. Biophys J. 1988 Nov;54(5):955–960. doi: 10.1016/S0006-3495(88)83031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Alkon D. L. Associative behavioral modification in hermissenda: cellular correlates. Science. 1980 Jul 18;209(4454):412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- Farley J., Alkon D. L. Associative neural and behavioral change in Hermissenda: consequences of nervous system orientation for light and pairing specificity. J Neurophysiol. 1982 Sep;48(3):785–807. doi: 10.1152/jn.1982.48.3.785. [DOI] [PubMed] [Google Scholar]
- Geiselman C. W., Burke C. N. Exact anhydride: epoxy percentages for araldite and araldite-epon embedding. J Ultrastruct Res. 1973 May;43(3):220–227. doi: 10.1016/s0022-5320(73)80034-6. [DOI] [PubMed] [Google Scholar]
- Goh Y., Lederhendler I., Alkon D. L. Input and output changes of an identified neural pathway are correlated with associative learning in Hermissenda. J Neurosci. 1985 Feb;5(2):536–543. doi: 10.1523/JNEUROSCI.05-02-00536.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W. T., Juraska J. M., Volkmar F. R. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979 Jul;26(3):287–297. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- Horn G., Rose S. P., Bateson P. P. Experience and plasticity in the central nervous system. Science. 1973 Aug 10;181(4099):506–514. doi: 10.1126/science.181.4099.506. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R., de Courten C., Garey L. J., Van der Loos H. Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neurosci Lett. 1982 Dec 13;33(3):247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Crow T., Alkon D. L. Change in a specific phosphoprotein band following associative learning in Hermissenda. Nature. 1981 Oct 22;293(5834):658–660. doi: 10.1038/293658a0. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Alkon D. L. Prolonged RNA changes in the Hermissenda eye induced by classical conditioning. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7800–7804. doi: 10.1073/pnas.85.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kusky J. R. Synapse elimination in the developing visual cortex: a morphometric analysis in normal and dark-reared cats. Brain Res. 1985 Sep;354(1):81–91. doi: 10.1016/0165-3806(85)90071-9. [DOI] [PubMed] [Google Scholar]
- Proctor W., Roper S. Competitive elimination of foreign motor innervation on autonomic neurones in the frog heart. J Physiol. 1982 May;326:189–200. doi: 10.1113/jphysiol.1982.sp014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Voyvodic J. T., Magrassi L., Yawo H. Nerve terminal remodeling visualized in living mice by repeated examination of the same neuron. Science. 1987 Nov 20;238(4830):1122–1126. doi: 10.1126/science.3685967. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J. P., Eckenhoff M. F., Zecevic N., Goldman-Rakic P. S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986 Apr 11;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M. R., Mollgaard K., Diamond M. C., Bennett E. L. Negative as well as positive synaptic changes may store memory. Psychol Rev. 1972 Jan;79(1):93–96. doi: 10.1037/h0031861. [DOI] [PubMed] [Google Scholar]
- Scheich H. Neural correlates of auditory filial imprinting. J Comp Physiol A. 1987 Sep;161(4):605–619. doi: 10.1007/BF00603664. [DOI] [PubMed] [Google Scholar]
- West A., Barnes E., Alkon D. L. Primary changes of voltage responses during retention of associative learning. J Neurophysiol. 1982 Nov;48(5):1243–1255. doi: 10.1152/jn.1982.48.5.1243. [DOI] [PubMed] [Google Scholar]