Abstract
Objectives
To assess the long-term outcomes and objective response to pre-radiation chemotherapy and radiation in adult high-risk medulloblastoma.
Methods
In this prospective phase II trial, adults with high-risk medulloblastoma were treated with three cycles of pre-radiation cisplatin, etoposide, cyclophosphamide, and vincristine followed by craniospinal radiation (CSI). Objective response (OR), progression free survival (PFS), overall survival (OS), and toxicities were assessed.
Results
Eleven patients were enrolled over a 6 year period. Six (55%) had subarachnoid metastases. Two (18%) had an OR to pre-radiation chemotherapy. Two (18%) progressed while on chemotherapy. Completion of CSI was not compromised. The OR rate after chemotherapy and radiation was 45 % (5/11). Non-evaluable patients at both time points weakened the response data conclusions. Median PFS was 43.8 months. Five year PFS was 27%. Five year OS was 55%. Non-metastatic (M0) and metastatic (M+) patients had similar outcomes.
Conclusions
The OR to this pre-radiation chemotherapy regimen is lower than anticipated from the adult and pediatric literature raising a question about comparative efficacy of chemotherapy in different age groups. The OS achieved is similar to retrospective adult series, but worse than pediatric outcomes. Although this regimen can be administered without compromising delivery of CSI, our results do not provide support for the use of this neoadjuvant chemotherapy for adult medulloblastoma.
Keywords: Adult Medulloblastoma, Pre-radiation chemotherapy
Introduction
Medulloblastoma in adults is rare, with an incidence of 0.5 per million per year.1 As a result, treatment strategies for adults with medulloblastoma are largely inferred from pediatric results. Prospective pediatric trials have established that gross residual disease at the primary tumor site after surgery and evidence of dissemination are independent negative prognostic factors. Disease staging for adults using similar criteria appears to have prognostic significance, distinguishing between standard- and high-risk groups, although not all reports confirm this observation, particularly with long-term follow-up.2,3,4,5 The addition of adjuvant chemotherapy for children with high risk medulloblastoma improves progression-free survival (PFS) and overall survival (OS).6,7,8 This strategy has frequently been applied in adult patients, but without prospective controlled trial evidence.
In recent years the molecular classification of medulloblastoma has advanced greatly and provided new insights into biological similarities and differences between pediatric and adult medulloblastoma.9,10,11,12,13 The issue of therapeutic inferences for adult medulloblastoma from pediatric results is becoming increasingly complex as the role of molecular subgroups in risk stratification increases.12,13 Since the ability to perform large-scale randomized clinical trials for adult medulloblastoma is hampered by small patient numbers, execution of smaller trials with fewer patients, generally using historical controls and often including comparison with pediatric medulloblastoma patients represents a possible “screening” strategy for various regimens.
E4397 was a phase II trial of pre-radiation chemotherapy for high-risk medulloblastoma in adults based closely on a pediatric regimen which included cisplatin (CDDP), etoposide (Etop), cyclophosphamide (Cyclo), and vincristine (Vcr) monthly for three cycles, followed by CSI.14,15,16 The primary aim of E4397 was to evaluate the objective response rate (OR) after pre-radiation chemotherapy and after completion of radiation. The secondary objectives were to evaluate progression free survival (PFS), overall survival (OS), and toxicities. This study was undertaken by the Eastern Cooperative Oncology Group (ECOG-ACRIN) and Southwestern Oncology Group (SWOG).
Materials and Methods
Patients greater than 18 years of age with histologically verified, newly diagnosed medulloblastoma with high-risk characteristics, defined as either > 1.0 cm2 residual tumor at the primary site after surgery, or evidence of subarachnoid dissemination (M+) were eligible for this study and are included in this report. Patients with supratentorial PNET and ependymoma with subarachnoid dissemination were also eligible and are summarized in the Appendix. (See Supplemental Material)
Initial staging included brain MRI obtained within 48 hours post-operatively to assess residual disease. MRI of the entire spine and CSF cytology were obtained following surgery prior to enrollment. All MRI scans were performed without and with gadolinium contrast. Imaging results were reported based on local interpretation.
Patients were required to have a stable corticosteroid dosage, no midline shift greater than 1 cm, and no intracranial pressure elevation. ECOG performance status could be 0 – 2. Adequate hematologic parameters (WBC > 4,000/mm3, platelets > 125,000/mm3, Hgb > 10gm) , no clinically significant serum chemistry abnormalities, liver enzymes < 2x normal, creatinine clearance >70 ml/min, and no history of pulmonary disease or DlCO > 60% predicted were required. No prior treatment with radiation or chemotherapy was allowed. Enrollment was to be between 10 and 21 days post surgery. Institutional IRB approval and informed consent were required.
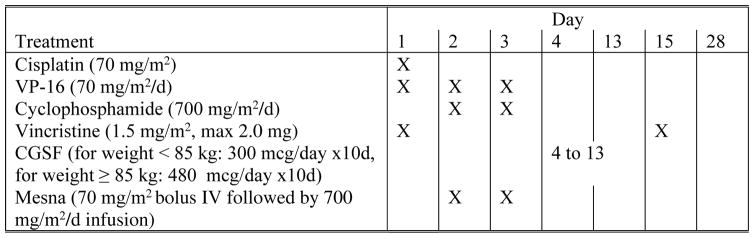
The chemotherapy plan utilized three 28 day cycles including: 1) CDDP 70 mg/m2 on day 1; 2) Etop 70 mg/m2 on days 1,2, and 3; 3) Cyclo 700 mg/m2 on days 2 and 3; and 4) Vcr 1.5 mg/m2 (maximum 2.0 mg) on days 1 and 14 of each cycle. Mesna, 70 mg/m2 bolus followed by 700 mg/m2 by continuous infusion over 24 hours following Cyclo was given. All patients received GCSF, 300 mcg per day for 10 days following each cycle. ( Figure 1)
Figure 1.
E4397 CHEMOTHERAPY REGIMEN
The treatment plan used in E4397 included three cycles of pre-radiation chemotherapy delivered at four week intervals. This was followed by radiation to a dose of 54 Gy to the primary tumor site and 36 Gy to the craniospinal axis, delivered in daily fractions of 1.8 Gy. Sites of obvious metastatic disease received an additional radiation boost.
CSI began four weeks following completion of chemotherapy. The radiation plan called for 54 Gy to the primary site and 36 Gy as the craniospinal dose with boosts to sites of obvious metastatic disease. A daily fraction size of 1.8 Gy was used for all sites.
Assessment of Response and Toxicity
For assessment of response to treatment, the brain MRI was repeated on day 28 of cycles 1 and 2 of chemotherapy, and the spine MRI was repeated if initially positive. Brain and total spine MRI were repeated prior to the start of radiation. Brain MRI was performed following the completion of radiation, then every 3 months for 2 years from study entry, then every 6 months for 5 years from study entry, and then annually. Total spine MRI was performed on the same schedule if it had ever been positive, or on a yearly basis. CSF cytology was obtained prior to the first cycle of chemotherapy, just prior to radiation, and at completion of radiation. Subsequent CSF testing was performed if new symptoms or radiographic changes occurred.
Assessments of the patient’s overall health, history and physical exam, neurological exam, and performance status were performed at baseline, day 28 of chemotherapy cycles 1 and 2, prior to radiation therapy, at completion of radiation therapy, every 3 months until 2 years from study entry, every 6 months until 5 years from study entry, and then annually. Hematologic evaluations were obtained at baseline, weekly during chemotherapy, prior to radiation therapy, at completion of radiation therapy, every 3 months until 1 year from study entry, then annually. Audiograms were performed prior to treatment and repeated only if grade 2 or greater tinnitus or hearing loss were reported.
Complete response (CR) was defined as disappearance of all disease demonstrated by MRI and negative CSF cytology. Persistence of small areas of T2 hyper-intensity without contrast enhancement at the primary tumor site, persistent meningeal enhancement at the operative site, or smooth diffuse dural enhancement were not scored as residual disease. Patients must have been off steroids, and response must have been sustained for > 4 weeks. Partial response (PR) was a decrease of at least 50% in the sum of the cross-section products of all measurable lesions. Steroid dose must have been stable or decreasing. Stable disease (SD) was no change, an increase of <25%, or a decrease of <50% in all measurable lesions, persistently negative cytology, and no new areas of meningeal enhancement. Progressive disease (PD) was an increase of >25% in any measurable lesion, the development of new lesions, either parenchymal or meningeal. The demonstration of persistently positive or newly positive CSF cytology following chemotherapy was considered PD.
New focal meningeal or subependymal enhancement demonstrated by MR scanning was considered evidence of PD even though those lesions were not often measurable. Diffuse dural enhancement in the absence of positive cytology or progressive clinical symptoms was not considered PD.
Toxicity summaries include all medulloblastoma patients who began protocol treatment. CTC version 2.0 was used.
Statistical Methods
The primary endpoint was to estimate the OR (CR + PR). Secondary endpoints included five year PFS and OS. Planned accrual was thirty-three patients. A stopping rule would have halted accrual if ≥7 of the first 10 patients were unable to complete 2 cycles of chemotherapy. PFS was defined as time from date of registration to evidence of progressive disease or death without progressive disease. Patients without evidence of progression were censored at the date last known to be free of progression. OS was defined as time from registration to death from any cause. Patients alive at time of current analysis were censored at the date last known to be alive. The Kaplan-Meier method was used to estimate PFS and survival distributions. Confidence intervals (CI) for median PFS and survival were calculated using the method of Brookmeyer and Crowley.17,18
Results
Patient Accrual
E4397 opened in July 1998 and closed in September 2004. Eleven medulloblastoma patients were enrolled for an accrual rate of 1.8 patients per year. The diagnosis of medulloblastoma was confirmed by central pathology review in 8 of 11 cases. The median age was 35 years with a range from 21 to 39 years (Table 1). Six of 11 patients (55%) were M+. The study was open at many ECOG and SWOG institutions however only ten institutions enrolled patients, and only three enrolled more than one patient. The study was closed primarily due to slow accrual.
Table 1.
PATIENT CHARACTERISTICS
| N = 11 (%) | |
|---|---|
| Gender | |
| Female | 6 (55) |
| Male | 5 (45) |
| Race | |
| White | 10 (91) |
| Other | 1 (9) |
| Age | |
| Minimum | 21 |
| 25th percentile | 23 |
| Median | 35 |
| 75th percentile | 39 |
| Maximum | 39 |
| ECOG Performance Status | |
| (0) Fully Active | 5 (45) |
| (1) Ambulatory | 3 (27) |
| (2) Self care, but not work | 3 (27) |
| Surgery | |
| Biopsy only | 2 (18) |
| Resection | 9 (82) |
| Metastases | |
| M0 | 5 (45) |
| M+ | 6 (55) |
Treatment Parameters
Eighty-two percent (9/11) of the medulloblastoma patients had a major resection. Two patients had biopsy only.
Ninety-one percent (10/11) of the medulloblastoma patients completed chemotherapy. One patient progressed after two cycles, and one progressed after the third cycle but prior to radiation.
Eighty-two percent (9/11) of the medulloblastoma patients completed CSI. One refused treatment beyond the 3 cycles of chemotherapy and 1 died just prior to RT from an acute cardiopulmonary event. Median primary tumor dose was 54 Gy (range 54.0 to 54.6). Median craniospinal dose was 36 Gy (range 36 to 45). Median interval from surgery to the start of radiation was 17.7 weeks (range 14.8 to 21.4). The median duration of RT was 6.5 weeks (range 5.0 to 7.0).
Toxicities
The pattern of toxicities was as anticipated from prior experience with this chemotherapy regimen and CSI.15,16,19 ( Table 2) Among 11 medulloblastoma patients six experienced grade 3 toxicities and four experienced grade 4 toxicities. These were predominantly hematologic toxicities, fatigue, nausea, vomiting, and anorexia. Two patients experienced fever or infection while neutropenic. Two patients experienced grade 4 radiation dermatitis. Minor electrolyte alterations were common but rarely significant. One patient experienced transient grade 4 renal toxicity. Two patients had mild hearing loss. One death occurred during treatment, but was not considered treatment related. This was suspected to be from a pulmonary embolus.
Table 2.
TOXICITY INCIDENCE for 11 MEDULLOBLASTOMA PATIENTS: CTC version 2.0 was used for toxicity grading.
| Grade | 1,2 (n) | 3 (n) | 4 (n) | 5 (n) | |
|---|---|---|---|---|---|
| Toxicity | |||||
| Hemoglobin | 9 | 1 | |||
| Leukocytes | 6 | 4 | 1 | ||
| Neutrophils | 3 | 1 | 4 | ||
| Platelets | 8 | 3 | |||
| Transfusions: Platelets | 1 | ||||
| Transfusions: pRBCs | 1 | ||||
| Febrile neutropenia | 1 | ||||
| Infection | 1 | 2 | |||
| Hypernatremia | 2 | ||||
| Hyponatremia | 3 | 1 | |||
| Hypokalemia | 2 | 1 | |||
| Hyperglycemia | 10 | ||||
| Hypoglycemia | 1 | ||||
| Hypercalcemia | 1 | ||||
| Hypocalcemia | 5 | ||||
| Hypermagnesemia | 1 | ||||
| Hypomagenesemia | 5 | ||||
| Creatinine | 1 | ||||
| Alkaline phosphatase | 5 | ||||
| SGOT | 3 | ||||
| Billirubin | 1 | ||||
| Fatigue | 7 | 3 | |||
| Weight Loss | 5 | ||||
| Weight Gain | 3 | ||||
| Anorexia | 3 | 3 | |||
| Dehydration | 1 | 1 | |||
| Dsyphagia | 3 | ||||
| Mucositis/Stomatitis | 4 | 1 | |||
| Nausea | 7 | 2 | |||
| Vomiting | 3 | 2 | 1 | ||
| Confusion | 1 | ||||
| Dizziness | 4 | ||||
| Syncope | 1 | ||||
| Anxiety/Agitation | 2 | 1 | |||
| Personality Change | 1 | ||||
| Memory Loss | 2 | ||||
| Tremor | 1 | ||||
| Blurred/Double Vision | 4 | ||||
| Hearing loss | 2 | ||||
| Neuropathy | 5 | 1 | |||
| Bone pain | 1 | ||||
| Headache | 5 | ||||
| Myalgia | 3 | ||||
| Arthralgia | 2 | ||||
| Irregular Menses | 3 | 1 | |||
| Radiation Dermatitis/Recall | 2 | ||||
| Alopecia | 10 | ||||
| Cushingoid Features | 2 | ||||
| WORST DEGREE | 1 | 6 | 4 |
Response Assessment
The OR at conclusion of chemotherapy for the 11 medulloblastoma patients was 18% (2/11) (.95CI:2.2%-51.7% ), with 1 CR and 1 PR. In three patients disease stabilized. Progression prior to starting radiation was observed in 18% (2/11). Four patients were not evaluable for response at the conclusion of chemotherapy including one on steroids, one who refused a spinal tap, one whose follow-up was by CT, and one with incomplete information.
OR at completion of chemotherapy and radiation was 45% (5/11) (.95CI:16.7%–76.2% ). Twenty-seven percent (3/11) achieved a CR, and 18% (2/11) achieved a PR. In 18% (2/11) disease stabilized. PD was seen in 9% (1/11). Three patients were not evaluable for response at the completion of all therapy including one patient who refused a spinal tap to confirm CR, one whose follow-up was by CT, and one with incomplete information.
Outcome
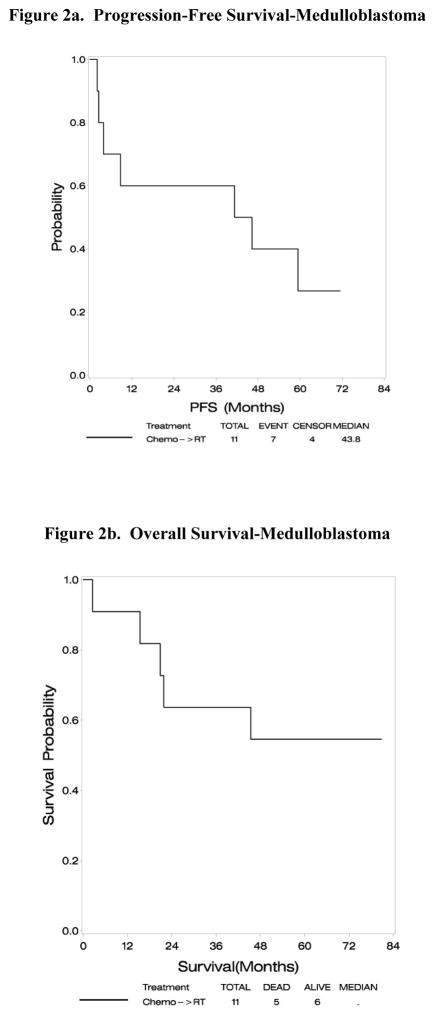
At the time of analysis the median follow up was 63 months. Median PFS was 43.8 months (95% CI:2.0-73+ (upper limit not reached as last patient censored at 71.3 months). Five of 11 patients have died. Median survival has not been reached. Five year OS was 55% and PFS was 27% (Figure 2). Median PFS was 41.2 months for the five M0 patients, and 59.3 months for six M+ patients. Five year OS was 60% and PFS 20% for M0 patients versus 50% OS and 30% PFS for M+ patients.
Figure 2.
Assessment of outcome in E4397 at a median follow-up of 63 months demonstrates a median PFS of 43.8 months (95%CI: 2.0-73+ (upper limit not reached as last patient censored at 71.3 months)), and a 5 year PFS of 27%. Median survival has not been reached. The 5 year OS is 55%.
Discussion
The SEER database of all medulloblastoma patients greater than 18 years of age shows 5- and 10-year survival rates of 64 and 50%, with a 10.6 year median survival.20,21 In series reporting long-term outcome for adults with high-risk disease the 5-year PFS ranges from 38 to 61% and 5-year OS ranges from 54 to 69%.2,4,22 The 5-year PFS of 27% observed in E4397 is lower than that reported in the literature. The 5-year OS of 55% in these 11 high risk patients is similar to those reports. Our results do not suggest an advantage in long-term outcome with this pre-radiation chemotherapy regimen. M+ patients do worse than M0 patients in most, but not all reports.13,22,23,24 In E4397 there was little difference in outcome between the M0 and M+ patients.
The chemotherapy response rate (OR) of 18% suggests a lower chemotherapy efficacy than had been anticipated from prior adult reports. However, this conclusion is limited by the small number of patients, including the fact that 4 patients were not evaluable for response at completion of chemotherapy. Galanis reported an 85% OR for adult patients using platinum-based regimens assessed retrospectively, many of whom were treated at recurrence.23 The sparse response data regarding upfront chemotherapy for high-risk medulloblastoma in the adult literature, totaling 12 patients, suggest an OR of greater than 50%.24,25
In pediatric phase II and III trials utilizing pre-radiation chemotherapy, response rates range from 29 to 74%. Progression prior to radiation is typically observed in about 20%, with reports ranging from 0 to 38%.8,14,15,26,27,28,29,30,31,32,33 The OR is lower and progression prior to radiation more likely in M+ children.27,28,29 Three pediatric randomized trials for high-risk medulloblastoma patients using pre-radiation CDDP/Cyclo-containing regimens, followed by CSI and maintenance chemotherapy, two of which were reported while E4397 was underway, did not demonstrate improved OS.8,29,30,32 The best results reported for pediatric high-risk medulloblastoma patients were achieved by Packer using CSI with concurrent Vcr and post-radiation CCNU, CDDP, and Vcr. The 5-year PFS was 85%, with M0 patients faring better than M+ patients.34 This regimen has not been as successful or as well tolerated in adults.23,24 Jakacki observed a 80% 5 year OS and 66% PFS for M+ children treated with carboplatin and vincristine concurrent with Cr-Sp RT, followed by maintenance chemotherapy.35
The only other prospective adult medulloblastoma trial was reported by Brandes, et al and included a total of 36 patients, of whom 26 had high-risk disease. They observed a 5-year OS of 73% and a 5-year PFS of 69% for high-risk patients.4,36 That cohort had a slightly shorter interval from surgery to radiation than in E4397 (13.9 vs 17.7 weeks), and risk categorization was not identical to E4397, using residual disease of >1.5 cm2 or M+ staging. The percentage of M+ patients in the Brandes trial was 60% and in E4397 was 55%. Thus the difference in results does not relate to the percentage of M+ patients. Of note, after a median follow-up of 7.6 years, M status and residual disease after surgery did not have a significant effect on PFS or OS, and the difference in outcome between low- and high-risk patients was no longer significant. This was attributed to late recurrences in the low-risk group.4,36
E4397 confirms the feasibility of delivering moderately intensive multi-agent chemotherapy prior to CSI without altering the planned radiation schedule. The median duration of radiation was 6.5 weeks. A more protracted radiation course has been associated with worse outcome.37 Delays in starting RT have been associated with poorer outcome in pediatric trials.8,28,29,30 In E4397 the median duration from surgery to initiation of radiation was 17.7weeks (range 14.8 to 21.4).
Conclusions drawn from E4397 are limited by small patient numbers, an inherent problem of looking at a subgroup of patients in an already rare disease. Multi-institutional studies are critical for trials of rare cancers, and the cooperative group setting is well-suited for this. E4397 was open at many ECOG-ACRIN and SWOG institutions, however only 10 accrued patients and most accrued only one patient. The rate of enrollment was 1.8 patients per year. The trial by Brandes et al. enrolled 2.16 high-risk medulloblastoma patients per year.4 At the institutional and the cooperative group level that accrual rate is too low to offset efforts required to maintain the study. These pressures contributed to the closure of E4397. One practical solution would be to utilize only institutions demonstrating a history of treating a set minimum number of patients per year who fit the eligibility criteria. Using a multinational intergroup format may also facilitate better accrual. Small patient numbers also magnify the effect of patient inevaluability for endpoints such as OR, and subsequent conclusions are weakened as a result. However, assessing response rates using only evaluable patients inappropriately inflates the OR. Promoting better accrual and requiring central review of response are two ways to improve future trials.
Evolution of therapies also has an impact on accrual to trials for rare diseases. Maintaining studies over extended periods often fails to keep up with evolving trends and newer therapies. This led to a substantial revision in the protocol utilized by Brandes et al.36 Similarly, the publication of negative neoadjuvant pediatric medulloblastoma trials while E4397 was in progress was another influential factor in the decision to close E4397.8,29
Given the paucity of outcome data in adult medulloblastoma, comparing the results of E4397 with those of the pediatric trials is valuable. However a number of issues that limit these comparisons deserve special attention including biological differences in the neoplasms of these two age groups, study design issues, and treatment differences. In regard to biological differences Group 3 and Group 4 have the poorest prognosis, 40% and 75% 5 years survival respectively, and both tend to metastasize. Group 3 is rare in adults, while Group 4 accounts for 25%. Of the more favorable subgroups WNT comprises 15% and SHH 60% of adult patients.9,13,38 Group 3 and Group 4 are likely overrepresented in high risk cohorts such as that of our trial. However, E4397 antedated the current molecular characterization of medulloblastoma, and unfortunately we are unable to retroactively assess these molecular characteristics.
Regarding trial design, clinical criteria for risk stratification are fundamental to medulloblastoma trials. At the time of development of E4397 the significance of residual disease was still being assessed with mixed reports in the early 1990s regarding its prognostic significance. In 1997 Albright published data now considered the consensus standard, indicating that residual disease > 1.5 cm2 assessed by CT or MRI was associated with higher risk of recurrence.8,39 Although our criteria for high-risk differed slightly from that standard, the use of a smaller cutoff (> 1.0 cm2) for post-operative residual disease does not appear to have biased the results towards a more favorable outcome. A recent observational report of adult patients with nonmetastatic medulloblastoma reported that patients with any residual tumor fared more poorly than those without.40 This issue continues to generate discussion as residual tumor was not a predictor of outcome in long-term follow-up by Brandes, et al.4,13
Another important design issue relevant to the pediatric-adult comparison is chemotherapy dosing. The dosing used in E4397 was lower than that used in pediatric trials of similar regimens by 30% for cisplatin, cyclophosphamide and etoposide. This was based on single institution experience that included children and adults using this regimen.15,16 While dosing differences weaken comparisons between the different age groups, the need for dose modification in adults is well known. Greenburg, et al. observed greater toxicities in adults treated with pediatric medulloblastoma chemotherapy protocols.24 Dose reductions are commonly required in both pediatric and adult post-radiation maintenance chemotherapy.34,40 Notably, the incidence of ototoxicity in E4397 was much lower than that seen in trials using post-radiation cisplatin. The incidence of neuropathy, largely attributed to vincristine, was also much lower.40
Even with these limitations the current trends in adult medulloblastoma therapy continue to be based heavily on inference from pediatric observations. For example, the use of lower dose CSI and adjuvant chemotherapy for standard risk adult medulloblastoma patients is now common despite the lack of validation in adults.41 To the extent that E4397 suggests a lower efficacy for chemotherapy in adults, these inferences must be made in a qualified manner.
Conclusions
E4397 demonstrates prospectively the feasibility and safety of moderately intensive pre-radiation chemotherapy for adults with high-risk medulloblastoma. This sequence of therapy did not interfere with completing CSI. The OS observed in E4397 is comparable with other reports for adult high risk medulloblastoma, however our results do not indicate a therapeutic advantage in utilizing this pre-radiation chemotherapy, although the incidence of ototoxicity and neuropathy was less. The percentage of M+ patients is higher in this trial and in the prospective trial by Brandes et. al. than in retrospective reports. However, there was little difference in outcome between M+ and M0 patients in our cohort. Viewed in conjunction with negative trials of pre-radiation chemotherapy in pediatric medulloblastoma, the standard sequence of CSI, typically including concurrent chemotherapy and post-radiation chemotherapy is favored for adult high-risk medulloblastoma patients; a recommendation still largely derived by inference from pediatric recommendations.
Although small patient numbers and the presence of inevaluable patients limit our conclusions, the comparatively low OR to pre-radiation chemotherapy raises an important question about the efficacy of chemotherapy in adult high risk medulloblastoma, thus raising concern about the validity of inferences from the pediatric experience to the management of adult medulloblastoma.
Despite the difficulties in completing trials in medulloblastoma, the value of these trials is increasing as we achieve a better understanding of the biological differences between pediatric and adult medulloblastoma. While biologically based risk-adapted treatments of pediatric medulloblastoma will continue to inform us on new directions for adult medulloblastoma, adult trials are necessary. The strong relation between targetable molecular abnormalities, for example SHH pathway abnormalities, and adult medulloblastoma requires that new trials include detailed molecular profiling and evaluate targeted therapies.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contribution of Dr Mark T. Jennings, M.D. in the development of the adult version of this chemotherapy regimen.
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA21115, CA23318, CA66636, CA180847, CA49957, CA189863, CA35267, CA180799, CA21076, CA180888, CA32102, CA180818, CA58861, CA180835, CA14028, CA180802, CA16116, CA180844, CA39229, CA180858, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
COI Statement: Dr. Mehta has served in the past as a consultant for Genentech, Novartis, with stock options in Pharmacyclics, and is on the Board of Directors of Pharmacyclics
References
- 1.Giordana MT, Schiffer P, Lanotte M, et al. Epidemiology of adult medulloblastoma. Int J Cancer. 1999;80:689–692. doi: 10.1002/(sici)1097-0215(19990301)80:5<689::aid-ijc10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Prados MD, Warnick RE, Wara WM, et al. Medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1995;32:1145–1152. doi: 10.1016/0360-3016(94)00476-2. [DOI] [PubMed] [Google Scholar]
- 3.Herrlinger U, Steinbrecher A, Rieger J, et al. Adult medulloblastoma: Prognostic factors and response to therapy at diagnosis and at relapse. J Neurol. 2005;252:291–299. doi: 10.1007/s00415-005-0560-2. [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Franceschi E, Tosoni A, et al. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110:2035–2041. doi: 10.1002/cncr.23003. [DOI] [PubMed] [Google Scholar]
- 5.Fellay CN, Frappaz D, Sunyach MP, et al. Medulloblastoma in adults: prognostic factors and lessons from pediatrics. Curr Opin Neurol. 2011;24:626–632. doi: 10.1097/WCO.0b013e32834cd4b1. [DOI] [PubMed] [Google Scholar]
- 6.Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy, with and without CCNU, vincristine and prednisone. J Neurosurg. 1990;72:572–582. doi: 10.3171/jns.1990.72.4.0572. [DOI] [PubMed] [Google Scholar]
- 7.Tait DM, Thornton-Jones H, Bloom HJG, et al. Adjuvant chemotherapy for medulloblastoma: the first multicentre control trial of the International Society of Pediatric Oncology (SIOP I) Eur J Cancer. 1990;26:464–469. [PubMed] [Google Scholar]
- 8.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children’s Cancer Group 921 randomized Phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 9.Korshunov A, Remke M, Wiebke W, et al. Adult and pediatric medulloblastoma are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28:3054–3060. doi: 10.1200/JCO.2009.25.7121. [DOI] [PubMed] [Google Scholar]
- 10.Northcott PA, Hielscher T, Dubuc A, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011a;122:231–240. doi: 10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011b;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandes AA, Franceshci E. Neuro-oncology: Genetic variation in pediatric and adult brain tumors. Nat Rev Neurol. 2010;6:653–654. doi: 10.1038/nrneurol.2010.176. [DOI] [PubMed] [Google Scholar]
- 13.Brandes AA, Bartolotti M, Marucci G, et al. New perspectives in the treatment of adult medulloblastoma in the era of molecular oncology. Critical Reviews in Oncology/Hematology. 2015;94:348–359. doi: 10.1016/j.critrevonc.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Eng J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 15.Jennings MT, Cmelak A, Johnson MD, et al. Differential responsiveness among "high risk" pediatric brain tumors in a pilot study of dose-intensive induction chemotherapy. Pediatr Blood and Cancer. 2004;43:46–54. doi: 10.1002/pbc.20043. [DOI] [PubMed] [Google Scholar]
- 16.Moots PL, Jennings MT, Bowen MG, et al. Multi-agent chemotherapy followed by craniospinal radiation for adults with “poor risk” medulloblastoma (MBL) and ependymoma with subarachnoid dissemination (D.E.) Neurology. 1998;50(Suppl 4):A380. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 19.Moots PL, O’Neill A, Barger GR, et al. Toxicities associated with chemotherapy followed by craniospinal radiation for adults with poor-risk medulloblastoma/PNET and disseminated ependymoma: a preliminary report of ECOG 4397. ASCO Annual Meeting; June 2004. [Google Scholar]
- 20.Lai R. Survival of patients with adult medulloblastoma. Cancer. 2008;112:1568–1574. doi: 10.1002/cncr.23329. [DOI] [PubMed] [Google Scholar]
- 21.Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors. Cancer. 2012;118:1313–1322. doi: 10.1002/cncr.26387. [DOI] [PubMed] [Google Scholar]
- 22.Padovani L, Sunyach MP, Perol D, et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 patients. Int J Radiat Oncol Biol Phys. 2007;68:433–440. doi: 10.1016/j.ijrobp.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Galanis E, Buckner JC, Schomberg PJ, et al. Effective chemotherapy for advanced CNS embryonal tumors in adults. J Clin Oncol. 1997;15:2939–2944. doi: 10.1200/JCO.1997.15.8.2939. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg HS, Chamberlain MC, Glantz MJ, et al. Adult medulloblastoma: multiagent chemotherapy. Neuro Oncol. 2001;3:29–34. doi: 10.1215/15228517-3-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spreafico F, Massimino M, Lorenza G, et al. Survival in adults treated for medulloblastoma using paediatric protocols. Eur J Cancer. 2005;41:1304–1310. doi: 10.1016/j.ejca.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Pendergass TW, Milstein JM, Geyer JR, et al. Eight drugs in one day chemotherapy for brain tumors: Experience in 107 children and rationale for pre-radiation chemotherapy. J Clin Oncol. 1987;5:1221–1231. doi: 10.1200/JCO.1987.5.8.1221. [DOI] [PubMed] [Google Scholar]
- 27.Mosijczuk AD, Nigro MA, Thomas PRM, et al. Pre-radiation chemotherapy in advanced medulloblastoma. A Pediatric Oncology Group Pilot Study. Cancer. 1993;72:2755–2762. doi: 10.1002/1097-0142(19931101)72:9<2755::aid-cncr2820720937>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Heideman RL, Kovnar EH, Kellie SJ, et al. Preirradiation chemotherapy with carboplatin and etoposide in newly diagnosed embryonal pediatric CNS tumors. J Clin Oncol. 1995;13:2247–2254. doi: 10.1200/JCO.1995.13.9.2247. [DOI] [PubMed] [Google Scholar]
- 29.Kortmann RD, Kuhl J, Timmermann B, et al. (2000) Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT ’91. Int J Radiat Oncol Biol Phys. 2000;46:269–279. doi: 10.1016/s0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 30.Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031) J Clin Oncol. 2013;31(23):2936–41. doi: 10.1200/JCO.2012.43.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strother D, Ashley D, Kellie SJ, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiation. J Clin Oncol. 2001;19:2696–2704. doi: 10.1200/JCO.2001.19.10.2696. [DOI] [PubMed] [Google Scholar]
- 32.Miralbell R, Fitzgerald TJ, Laurie F, et al. Radiotherapy in pediatric medulloblastoma. Quality assessment of Pediatric Oncology Group Trial 9031. Int J Radiat Oncol Biol Phys. 2006;64:1325–1330. doi: 10.1016/j.ijrobp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Verlooy J, Mosseri V, Bracard S, et al. Treatment of high risk medulloblastoma in children above the age of 3 years: A SFOP study. Eur J Cancer. 2006;42:3004–3014. doi: 10.1016/j.ejca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and Cisplatin, CCNU and vincristine chemotherapy. J Neurosurg. 1994;81:690–698. doi: 10.3171/jns.1994.81.5.0690. [DOI] [PubMed] [Google Scholar]
- 35.Jakacki IR, Burger PC, Zhou T, et al. Outcome of children with medulloblastoma treated with carboplatin during craniospinal radiotherapy: A Children’s Oncology Group Pahse I/II study. J CLin Oncol. 2012;30:2648–2653. doi: 10.1200/JCO.2011.40.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandes AA, Erani M, Amista P, et al. The treatment of adults with medulloblastoma: a prospective study. Int J Rad Onc Biol Phys. 2003;57:755–761. doi: 10.1016/s0360-3016(03)00643-6. [DOI] [PubMed] [Google Scholar]
- 37.Chan AW, Tarbell NJ, Black PM, et al. Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery. 2000;47:623–631. doi: 10.1097/00006123-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Samkari A, White JC, Packer RL. Medulloblastoma: Toward biologically based management. Semin Ped Neurol. 2015;22:6–13. doi: 10.1016/j.spen.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Albright AL, Wisoff JH, Zeltzer PM, et al. Effects of medulloblastoma resections on outcome in children: A report from the Children’s Cancer Group. 1996;38:265–271. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Friedrich C, von Bueren AO, von Hoff K, et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: A prospective observational multicentre study. Eur J Cancer. 2013;49:893–903. doi: 10.1016/j.ejca.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.