Abstract
Reading, an essential life skill in modern society, is typically learned during childhood. Adults who can read show white matter differences compared to adults who never learned to read. Studies have not established whether children who can read show similar white matter differences compared to children who cannot read. We compared 6-year old children who could decode written English words and pseudowords (n=31; Readers) and 6-year old children who could not decode pseudowords and had a standard score < 100 on a task for reading single words (n=11; Pre-Readers). We employed diffusion MRI and tractography to extract fractional anisotropy (FA) along the trajectory of 6 bilateral intra-hemispheric tracts and 2 posterior subdivisions of the corpus callosum. Readers demonstrated significantly increased FA within the left anterior segment of the superior longitudinal fasciculus (aSLF-L) and the right uncinate fasciculus (UF-R) compared to Pre-Readers. FA in the aSLF-L was significantly correlated with phonological awareness; FA in the UF-R was significantly correlated with language. Correlations in the UF-R but not the aSLF-L remained significant after controlling for reading ability, revealing that UF-R group differences were related to both children's language and reading abilities. Taken together, these findings demonstrate new evidence showing that individual differences in white matter structure relate to whether children have begun to read.
Keywords: diffusion MRI, reading development, tractography, white matter
Reading is one of the most important skills children must learn in modern societies. In the United States, children are typically first taught to read when they begin formal schooling, usually between 5 and 6 years of age. Through continual practice and exposure to print and speech, children learn how to link orthographic to phonological information in order to decode words and extract meaning from written text.
Reading abilities in adults and children are supported by a distributed network of cortical areas and the white matter pathways that connect them (Deheane 2009; Vandermosten, Boets, Wouters, et al. 2012; Wandell and Yeatman 2013). White matter is thought to support reading by rapidly transmitting neural signals amongst cortical areas involved in processing phonological, linguistic, and orthographic information (Ben-Shachar et al. 2007; Deheane 2009; Price 2012; Vandermosten, Boets, Wouters, et al. 2012; Wandell and Yeatman 2013). Reading involves both dorsal pathways, including the superior longitudinal fasciculus and arcuate fasciculus, and ventral pathways, including the inferior longitudinal fasciculus, the inferior fronto-occipital fasciculus, and the uncinate fasciculus (Catani et al. 2005; Ben-Shachar et al. 2007; Deheane 2009; Vandermosten, Boets, Wouters, et al. 2012; Wandell and Yeatman 2013; Welcome and Joanisse 2014; Cummine et al. 2015). The dorsal pathways connect the inferior parietal and posterior temporal cortex with the inferior frontal cortex and are thought to be involved in phonological processes for mapping sound to articulatory-based representations (Hickok and Poeppel 2004; Yeatman et al. 2011; Vandermosten, Boets, Wouters, et al. 2012; Brauer et al. 2013; Monzalvo and Dehaene-Lambertz 2013). The inferior longitudinal fasciculus, a ventral pathway, connects the occipital to temporal lobes and in language processing is thought to be involved in mapping sound to meaning (Hickok and Poeppel 2004). The inferior fronto-occipital fasciculus and uncinate fasciculus, also both ventral pathways, connect occipital and anterior temporal cortices to ventral prefrontal cortices, and are thought to be involved in mapping orthography to semantics (Vandermosten, Boets, Wouters, et al. 2012).
Diffusion MRI is currently the most common method for assessing white matter structure in relation to reading abilities in adults and children. In addition to the dorsal and ventral pathways described above, diffusion imaging studies have also implicated several additional white matter tracts in reading related processes. Such pathways include posterior callosal fibers (Odegard et al. 2009), specifically those that project to the temporal lobes, (Dougherty et al. 2007) and the corticospinal tract (Beaulieu et al. 2005; Deutsch et al. 2005; Niogi and McCandliss 2006)
A recent dMRI study compared adults who learned to read (either as children or as adults) to adults who never acquired reading skills, principally because of societal limitations to their exposure to education (Thiebaut de Schotten et al. 2014). Literate adults showed increased fractional anisotropy (FA), compared to the “ex-illiterate” and “illiterate” group, within the posterior segment of the left arcuate fasciculus, alternatively referred to as the indirect posterior segment of the superior longitudinal fasciculus, which connects ventral temporal and inferior parietal cortices (Martino et al. 2013). The results were interpreted as evidence of increased structural coherence in this pathway as the result of having learned to read. Earlier studies using T1-weighted volumetric approaches had found evidence for significant reductions in the size and volume of white matter tracts in an adult illiterate group compared to an adult reader group (Castro-Caldas et al. 1999; Carreiras et al. 2009); these volumetric differences were found in the splenium of the corpus callosum.
Developmentally, individual variations in diffusion properties of dorsal and ventral white matter pathways have been found to be associated with phonological skills in pre-reading children (Saygin et al. 2013; Vanderauwera et al. 2015; Vandermosten et al. 2015) and with reading skills in older children who had learned to read (Nagy et al. 2004; Beaulieu et al. 2005; Deutsch et al. 2005; Niogi and McCandliss 2006; Odegard et al. 2009; Rimrodt et al. 2010; Yeatman et al. 2011; Gullick and Booth 2014; Gullick and Booth 2015). Diffusion properties have also been observed to change in association with improvements in children's reading abilities following an intensive behavioral training for reading (Keller and Just 2009) and to predict later reading outcomes in children with dsylexia (Hoeft et al. 2007). Developmental changes in diffusion properties of the left arcuate fasciculus and left inferior longitudinal fasciculus have been associated with later reading skills in typically developing children with a wide range of reading abilities (Yeatman, Dougherty, Ben-Shachar, et al. 2012). However, studies have yet to establish whether white matter properties differ on the basis of whether children have begun to read at the earliest stages of reading education.
This study sought to establish whether young children who had begun to read at the initial stages of their formal education would show white matter differences compared to children of the same age who had not begun to read. We addressed this question by dividing a sample of healthy 6-year old children into two groups: children who could accurately decode pseudowords, thereby demonstrating that they had acquired an understanding for orthographic to phonological mapping, were classified as ‘Readers’; children who could not decode pseudowords were classified as ‘Pre-Readers’. We compared children in terms of demographic variables and in terms of their cognitive and linguistic abilities. All of the participants underwent dMRI and the scans were analyzed using tractography. We predicted that children in the Reader as compared to the Pre-Reader group would demonstrate structural white matter differences within white matter pathways associated with reading. Based on previous dMRI findings in adults, we evaluated the posterior segment of the superior longitudinal fasciculus (Thiebaut de Schotten et al. 2014). Based on dMRI studies of reading and white matter in children, we evaluated the anterior SLF and the arcuate fasciculus, the inferior longitudinal fasciculus, the corticospinal tract, the uncinate fasciculus, and posterior subdivisions of the splenium of the corpus callosum that contain fiber pathways projecting to temporal or occipital cortices. (Nagy et al. 2004; Beaulieu et al. 2005; Deutsch et al. 2005; Niogi and McCandliss 2006; Frye et al. 2008; Qiu et al. 2008; Odegard et al. 2009; Yeatman et al. 2011; Yeatman, Dougherty, Ben-Shachar, et al. 2012; Saygin et al. 2013; Vanderauwera et al. 2015; Vandermosten et al. 2015). In tracts where we identified group differences, we planned to conduct correlation analyses between white matter properties and specific reading-related skills in order to interrogate the possible sources of individual variability that may have contributed to the observed group differences in white matter structure.
Until now, most development studies of early readers and pre-readers have primarily identified white matter differences on the basis of whether children have specific behavioral and familial risk factors for reading disorders (Saygin et al. 2013; Vandermosten et al. 2015). Here, evidence for white matter differences between Readers and Pre-Readers would demonstrate that white matter differences at these ages would reflect whether children had acquired the ability to read. Overall, evidence for white matter differences at these ages is expected to inform understandings for the time course of white matter development within the context for how reading is typically acquired during childhood in modern societies.
Materials and Methods
Participants
Forty-five children, between 5 years and 10 months and 6 years and 8 months of age, were recruited for this study as part of a longitudinal study investigating the neural bases of reading in children born preterm or full term. All participants in the current study were born full term (≥36 weeks gestational age) and met the following eligibility criteria: monolingual American English speakers or bilingual speakers with at least 2 years of English exposure at daycare, pre-school and/or school, had standard scores > 70 on measures of nonverbal and verbal IQ, as measured by the Wechsler Abbreviated Scale of Intelligence 2nd edition (WASI-II (Wechsler and Hsiao-pin 2011)); were physically healthy with no history of neurological disorders or sensory impairments. The present sample was recruited through online parent groups, postings in local school newsletters and letters to families who had participated in past research studies in affiliated research laboratories at Stanford University. In order to enroll children with a broad range of reading abilities, recruitment materials did not mention reading skills as a study goal. Children born preterm were excluded from the present analyses given previous evidence for group differences in fractional anisotropy (FA) within certain pathways examined in the present study, including the inferior longitudinal fasciculus, uncinate, and corticospinal tracts (Groeschel et al. 2014; Travis, Adams, et al. 2015) and evidence for distinctive patterns of associations between cerebral white matter diffusion properties and reading abilities in children and adolescents born preterm or full term (Feldman et al. 2012). The experimental protocol was approved by the Stanford University Institutional Review Board. A parent or legal guardian provided informed written consent. Participants were compensated for participation.
All participants performed a detailed battery of behavioral testing (described below) and took part in an anatomical MRI scan that included T1 weighted and diffusion weighted sequences. Of the 45 full term participants recruited, three participants were not included in the present analyses because the children were either unable to perform MRI scans (n = 2) or moved too much during scanning (n = 1). Methods for how scans were assessed for head motion are described in a subsequent section. The final sample thus consisted of 42 subjects (15 males, mean age = 6.2 years ± 2.3 months).
Group status (Reader versus Pre-Reader) was determined based on the child's performance on the Word Attack Subtest of the Woodcock Reading Mastery Test, 3rd edition (WRMT-III) (Woodcock 2011). In this untimed test, children were asked to read aloud a list of pseudowords in order of increasing difficulty. We defined reading status using raw scores on a pseudoword reading task in order to identify those children who were able to decode words through grapheme-phoneme correspondences, a core skill in reading development (Gough and Tunmer 1986; Share 1995; Kirby et al. 2008), and to minimize inclusion of children who may have been using heuristics to identify familiar looking words. We set the threshold for Readers as ≥ 2 pseudowords read correctly (equivalent to a standard score ≥ 100), and for Pre-Readers as ≤ 1 pseudowords read correctly (equivalent to a standard score < 100). Using this approach we identified 11 Pre-readers (5 males) and 31 Readers (10 males). Divided in this way, the groups also differed in their scores on the Word Identification Subtest of the WRMT-III (Woodcock 2011), an untimed test of sequential word reading in order of increasing difficulty. Readers had a standard score > 100 on the Word Identification test whereas Pre-Readers had a standard score of < 100. Thus, though it was possible that the Pre-Readers may have used sight word vocabulary for reading, in this sample the children were weak on both pseudoword and real word reading.
Socio-economic status was measured with the 4-Factor Hollingshead Index (Hollingshead 1975), a weighted composite of both parents’ education level and occupation. At the time of participation, children in the Reader group were either enrolled in kindergarten or first grade. Participants were considered to have a family history of reading disability if a parent reported diagnosed or suspected reading problems in one or more immediate biological family member, including mother, father, or siblings (Snowling et al. 2012). Because of the high proportion of bilingual children in the Bay Area of California, we did not exclude children based on bilingual status but included such children only if they had been enrolled in an English-speaking daycare, preschool, or school for a minimum of 2 years and were not classified at school as an English Language Learner (ELL). Children were classified as bilingual if parents reported that children could conduct conversation fluently in another language besides English. Handedness was measured by the Edinburgh Handedness Inventory (Oldfield 1971).
Cognitive Measures
Participants completed a battery of cognitive tests to characterize their abilities in real word and pseudoword reading, phonological processing (including phonological awareness and phonological memory), rapid automatic naming, receptive and expressive language skills and general intelligence (see below for a detailed description of tasks and test batteries used).
Phonological Awareness
was assessed using three subtests of the Comprehensive Test of Phonological Processing (CTOPP; (Wagner et al. 1999): (1) In the Elision subtest, children are asked to remove a phoneme from a spoken word to form a new spoken word (e. g., “say tiger without saying /g/ ” = “tire”); (2) In the Sound Matching subtest, children are asked to identify a picture of an object whose name either begins or ends with a given sound (e. g., “which word starts with the same sound as ‘pan’: pig, hat or cone?”) (3) In the Blending Words subtest, children are asked to combine sounds to form a word (eg., /m/-/ă/-/d/ = “mad”).
Phonological Memory
or verbal short term memory, was assessed using two subtests of the CTOPP: (1) In the Memory for Digits subtest, children are asked to repeat a list of spoken digits, while the number of spoken digits increases from trial to trial; (2) In the Non-word Repetition subtest, children are asked to repeat a list of spoken non-words, while the number of syllables in each non-word increases from trial to trial.
Rapid Automatic Naming
was assessed using the Objects and Colors subtests of the CTOPP. In these subtests children are asked to name aloud, as fast as possible, familiar objects (e.g., “house”) or colored squares (“blue”) which are presented visually as large arrays of icons.
Expressive and Receptive Language Skills
were assessed with the Clinical Evaluation of Language Fundamentals 4th ed. (CELF-4; (Wigg et al. 2003)) and summarized using the Core Language composite score. The Core Language composite score consists of four subtests of the CELF-4: (1) The Concepts and Following Directions subtest, which assesses children's listening comprehension skills by asking children to point to pictures based on structural relations between the objects (e.g., “point to the small white ball that is next to a black house”). (2) The Word Structure subtest, which assesses children's knowledge of inflectional and derivational word morphology by having children fill in the blank in a sentence given the base morpheme of the word. (3) The Recalling Sentences subtest, which assesses children's working memory for sentences, by asking children to repeat spoken sentences of increasing word length and grammatical complexity and (4) The Formulating Sentences subtest, which assesses children's semantic and grammatical speech production abilities by having them generate a novel sentence using a target word.
Verbal and Nonverbal Intelligence
General verbal and non-verbal intellectual abilities were assessed using the Wechsler Abbreviated Scale of Intelligence (WASI-II), a nationally standardized test of general intellectual abilities (Wechsler and Hsiao-pin 2011). The Verbal Intelligence Index was assessed with two subtests of the WASI-II: (1) the Vocabulary subtest, which assesses verbal concept formation and verbal production skills by having children provide a conceptual definition for a word verbally and (2) the Similarities subtest, which assesses children's abstract verbal reasoning abilities by having children verbally describe how two items are conceptually related. Non-Verbal IQ was assessed with two additional subtests of the WASI-II: (1) the Matrix Reasoning subtest, which assesses children's abstract problem solving, inductive reasoning, and spatial skills by asking children to select a picture that is most consistent with a set of visually related objects and (2) the Block Design subtest, which assesses children's spatial perception, visual abstract reasoning, and problem solving by asking children to replicate a pattern by manually manipulating a set of colored blocks. The Full Scale IQ is based on all four subtests.
MRI Acquisition
MRI data were acquired on a 3T Discovery MR750 scanner (General Electric Healthcare, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) at the Center for Cognitive and Neurobiological Imaging at Stanford University (www.cni.stanford.edu).
High-resolution T1-weighted anatomical images were collected for each subject using a 5-minute inversion recovery (IR)-prep 3D fast-spoiled gradient (FSPGR) sequence collected in the sagittal plane (0.9mm cubed voxel size). This T1-weighted image was used as a common reference for alignment of the diffusion tensor image (DTI) maps.
dMRI data were acquired with a 5-minute diffusion-weighted, dual spin-echo, echo-planar imaging sequence with full brain coverage. Diffusion weighting gradients were applied at 30 non-collinear directions. We collected 70 axial slices in each participant (FOV = 240mm; 256 × 256, voxel size of 0.9375 × 0.9375 × 2 mm). Diffusion gradient strength was set to a b-value of 1,000 sec / mm2. In addition, 3 volumes were acquired at b=0 in the beginning of each scan.
Data Preprocessing
The T1 images were first aligned to the canonical ac-pc orientation. Diffusion weighted images were pre-processed with open-source software, mrDiffusion (http://white.stanford.edu/newlm/index.php/MrDiffusion) implemented in MATLAB R2012a (Mathworks, Natick, MA). The dual-spin echo sequence used here greatly reduces eddy-current distortions (Reese et al. 2003). For this reason, we did not need to correct for eddy current distortions. Subjects’ motion during the diffusion-weighted scan was corrected using a rigid body alignment algorithm (Rohde et al. 2004). Each diffusion weighted image was registered to the mean of the three non-diffusion (b0) images and the mean b0 image was registered automatically to the T1 image, using a rigid body transformation (implemented in SPM8, http://www.fil.ion.ucl.ac.uk/spm/; no warping was applied). The combined transform that resulted from motion correction and alignment to the T1 anatomy was applied to the raw data once, and the transformed images were resampled to 2 × 2 × 2mm isotropic voxels. Diffusion gradient directions were then adjusted to fit the resampled diffusion data (Leemans and Jones 2009).
For each voxel in the aligned and resampled volume, tensors were fit to the diffusion measurements using a standard least-squares algorithm. A continuous tensor field was estimated using trilinear interpolation of the tensor elements. The eigenvalue decomposition of the diffusion tensor was calculated and the resulting three eigenvalues (λ1, λ2, λ3) were used to compute fractional anisotropy (FA), radial diffusivity (RD, i.e., the mean of λ2 and λ3) and axial diffusivity (AD, i.e., λ1) (Basser and Pierpaoli 1996).
Head Motion
All dMRI scans were rigorously assessed for head motion using the following procedures. First, a trained research assistant (JNA) visually inspected raw images corresponding to each volume in the dMRI sequence and noted those volumes with obvious motion artifacts (typically observed as geometric distortions and stripes, and blurring signs). Second, we quantified the amount of translational head motion detected during image preprocessing for each diffusion volume. This procedure was achieved by assessing the magnitude (in millimeters) of motion correction required for each image volume along each plane. Based on these assessments, we chose to exclude image volumes from analyses only if visual inspection revealed obvious motion artifacts and/or translational head movement exceeded 4mm (2 voxels) in any direction. Using these criteria, one scan was determined to be unusable due to excessive head motion (29 volumes exceed 4mm translational motion). This subject was excluded from the current study. No volumes were excluded for any participant in the Pre-Reader group. In the Reader group, volumes were excluded in 2 children (one volume and 8 volumes, respectively).
Tractography
In order to maximize sensitivity while taking into account considerable individual variability, particularly at this early stage in brain development, our approach used individual tract identification in the native space of each child, followed by quantification of diffusivity properties along the length of the tract. Automatic tract segmentation and quantification was implemented using the Automated Fiber Quantification (AFQ; https://github.jyeatman/AFQ) software package and MATLAB. We identified 6 bilateral intra-hemispheric fiber tracts and 2 subdivisions of the corpus callosum that have been previously implicated in reading processes, as seen in Figure 1 (Ben-Shachar et al. 2007; Deheane 2009; Vandermosten, Boets, Wouters, et al. 2012; Wandell and Yeatman 2013). Specifically, we identified three segments of the superior longitudinal fasciculus (SLF), consistent with the terminology in (Martino et al. 2013): the long segment of the SLF, comprised of fronto-temporal fibers, also known as the Arcuate Fasciculus (Arc), the anterior segment of the SLF, comprised of fronto-parietal fibers (aSLF), and the posterior segment of the SLF (pSLF), comprised of temporo-parietal fibers. This division follows the anatomical segmentation scheme proposed by (Catani et al. 2005) for segmenting the arcuate fasciculus, but restricts the use of the term “arcuate” to the arc-shaped fronto-temporal fibers only, as in (Martino et al. 2013). We further identified the Uncinate Fasciculus (UF), the Corticospinal Tract (CST) and the Inferior Longitudinal Fasciculus (ILF). Finally, we identified two posterior subdivisions of the corpus callosum: callosal tracts connecting the temporal lobes (CC-Temp) and callosal tracts connecting the occipital lobes (CC-Occ). We selected all of these pathways a priori due to their known role in reading and reading development (Nagy et al. 2004; Beaulieu et al. 2005; Deutsch et al. 2005; Niogi and McCandliss 2006; Odegard et al. 2009; Rimrodt et al. 2010; Yeatman et al. 2011; Vandermosten, Boets, Wouters, et al. 2012; Saygin et al. 2013; Gullick and Booth 2014; Gullick and Booth 2015; Vanderauwera et al. 2015; Vandermosten et al. 2015).
Figure 1.
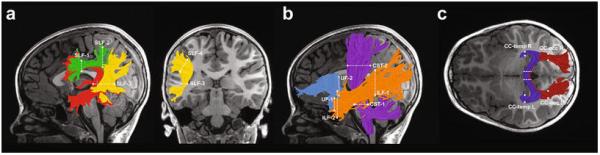
Tractography of 6 bilateral cerebral white matter tracts and 2 posterior segments of the corpus callosum. Left hemisphere cerebral tract renderings are displayed on a mid-sagittal T1 image from a representative child in the Reader group. Right hemisphere tract renderings not shown. Dashed lines represent the location of the regions of interest (ROIs) used to segment each cerebral tract from the whole-brain fiber group. Panel a illustrates the following subdivisions of the superior longitudinal fasciculus (SLF): Arcuate Fasciculus (Arc) = red, anterior SLF (aSLF) = green, and posterior SLF (pSLF) = yellow. Fibers belonging to the Arc were required to pass between SLF-1 and SLF-3. Fibers belonging to the aSLF were required to pass between SLF-1 and SLF-2. Fibers belonging to the pSLF were required to pass between SLF-3 and SLF-4 but not SLF-1. Panel b illustrates the following tracts: Inferior Longitudinal Fasciculus (ILF) = orange; Corticospinal Tract (CST) = purple; Uncinate Fasciculus (UF) = light blue. Panel c illustrates the following subdivisions of the corpus callosum (CC): temporal (CCTemp) = dark blue and occipital (CC-Occ) = dark red.
AFQ uses a three-step procedure to identify each tract in each individual: whole brain tracking, automatic segmentation using template ROIs warped to native space, and automatic refinement and cleaning (Yeatman, Dougherty, Myall, et al. 2012). First, whole-brain tractography was performed using a deterministic streamlines tracking algorithm (STT) (Conturo et al. 1999; Mori et al. 1999; Chang et al. 2005), with a fourth-order Runge-Kutta path integration method (Press et al. 2002). In the present study, the fiber tracking algorithm was seeded from each voxel in a white matter mask (FA>0.2 across the entire brain volume). Tracking proceeded in all directions and stopped when FA dropped below 0.15 or when the angle between the extension of a line in the direction of the current step and the direction of the next step was greater than 30°.
Second, fiber tract segmentation was done based on way-point regions of interest (ROIs). ROIs were defined on a template in MNI space according to published protocols for segmenting intra-hemispheric tracts (Wakana et al. 2004) and callosal segements (Huang et al. 2005). Then, a non-linear transformation (Friston and Ashburner 2004) was applied to warp these ROIs from the MNI template space into each individual's native space. In the way-point ROI procedure, each fiber from the whole-brain fiber group becomes a candidate for a specific fiber group if it passes through two ROIs that define the trajectory of the fiber group (Figure 1). This procedure was used to segment all tracts of interest, except for the pSLF. To segment the pSLF, we employed a modified two ROI waypoint procedure included as part of the AFQ software package (version 1.2; (Yeatman et al. 2014)). This procedure employs an additional “not” ROI (see Figure 1a) to exclude fibers that project anteriorly towards the frontal lobes and are likely to belong to the arcuate fasciculus. This procedure thus restricts the pSLF to only those fibers projecting vertically between a parietal ROI and a temporal ROI (Figure 1a).
Third, fiber tract refinement was done by comparing each candidate fiber to an established fiber tract probability map (Hua et al. 2008) and removing candidate streamlines that pass through regions of the white matter having a low probability for belonging to the tract. The core of the tract was calculated by defining 30 sample-points along the tract and computing the robust mean position of the corresponding sample points. The robust mean was computed by estimating the 3-dimensional Gaussian covariance of the sample points and removing fibers that are either located more than 5 standard deviations away from the mean position of the tract, or that differed more than 4 standard deviations in length from the mean length of the tract. This computation constituted the final automatic cleaning stage of the segmented tracts.
Fiber renderings for each tract and each individual were visually inspected prior to any knowledge of the individual's group status (Pre-Reader versus Reader) and prior to any statistical analyses, to ensure that each tract conformed to anatomical norms. Based on this inspection, we concluded that the automatic cleaning phase should be modified for two tracts: the aSLF and the pSLF. Both included many fibers that went through the waypoint ROIs but whose endpoints were not in the proper cortical positions (inferior frontal and inferior parietal for aSLF; inferior parietal and ventral posterior temporal for the pSLF). To improve the segmentation in terms of their anatomical endpoints, we adjusted the parameters of the automatic cleaning phase by incrementally reducing the cleaning parameters (automatically restricting the distance of streamlines from the core of the tract and restricting the length of the segments compared to mean length), and visually inspected the resulting tract rendering in each individual for anatomical correctness. For the left and right aSLF, we were able to remove fibers with anatomically incorrect endpoints by adjusting the cleaning parameters to exclude fibers if they were located more than 4 standard deviations from the mean position of the tract or if they were more than 1 standard deviation in length from the mean length. For the left and right pSLF, we removed fibers with anatomically incorrect endpoints by adjusting the cleaning parameters to exclude fibers if they were located more than 3 standard deviations in length from the mean length (the distance parameter was kept at 5, the default value).
Using these procedures, we were able to identify all 6 tracts, bilaterally, and the 2 subdivisions of the corpus callosum, in the majority of subjects, with the exception of the Arc-R, which could not be identified in 5 children (~16%) in the Reader group and in 3 children (~27%) in the Pre-Reader group. Visual inspection also revealed that the Arc-L in one child in the Reader group did not conform to anatomical norms and so this subject was excluded from analyses involving this tract. We attribute the difficultly in identifying the Arc-R to limitations of deterministic tractography approaches that cannot account for higher tract curvature and increased partial voluming with the aSLF-R, a finding consistent with several other reports (Catani et al. 2007; Lebel and Beaulieu 2009; Mishra et al. 2010; Yeatman et al. 2011; Travis, Adams, et al. 2015). Because we could only identify the Arc-R in 9 children in the Pre-Reader group, we chose to remove the Arc-R from group analyses. We were also unable to obtain reliable tracking of the pSLF-R in one child in the Reader group and in one child in Pre-Reader group. Fiber tracking was successful for the remaining pathways in all participants.
Fiber Tract Quantification: Tract Profiles
For each of the 6 bilateral intra-hemispheric tracts, diffusion properties (FA, RD, AD) were calculated at 30 equidistant nodes along a central portion of each fiber tract bounded by the same two ROIs used for tract segmentation (see below for a different method applied to the CC subdivisions). This procedure generated, for every tract and every individual, an FA tract profile that described the variations in FA along the central portion of the tract. At each node, diffusion properties were calculated by taking a weighted average across all fibers belonging to this tract. Each fiber's contribution to the average was weighted by the probability that a fiber was a member of the fascicle, computed as the Mahalanobis distance from the tract core (Yeatman, Dougherty, Myall, et al. 2012). This procedure minimizes the contribution of fibers located further from the fiber tract core that are more likely to be affected by adjacent tracts, and so minimizes the effect of partial voluming on diffusion estimates.
Fiber Tract Quantification: Corpus Callosum
In the occipital and temporal subdivisions of the CC, we used a different approach for quantification. We restricted the assessment of these tracts to the mid-sagittal plane (following (Dougherty et al. 2007)). This approach capitalizes on the high level of directional coherence that characterizes the mid-sagittal segment of CC fibers; it yields high reliability of diffusivity and anisotropy measures extracted from that segment. The CC is unique in providing an anatomically specified region of high directional coherence (Dougherty et al. 2005). We therefore extracted the mean FA values from a 1-centimeter long segment of each CC subdivision, extending 5 millimeters to the left and to the right of the mid-sagittal plane. This procedure was done for each subdivision and for each participant, separately. Diffusion measures in the CC were thus summarized as a single mean measure for each participant and subdivision.
Statistical Analyses
Group Comparisons: Demographic Variables and Cognitive Measures
Age standardized scores were calculated for each cognitive measure. Cognitive scores were classified as outliers if they differed by 3 standard deviations or more from the mean of the full sample (N=42). Based on this criterion, one child in the Pre-Reader group was found to be an outlier on tasks used to assess phonological awareness and expressive and receptive language abilities. All group analyses described below were repeated excluding this subject to assure that the reported group differences were not driven by this subject. Chi-square tests and two-tailed t-tests for independent samples were used to examine differences between the Reader and Pre-Reader groups on the basis of demographic variables and cognitive measures for reading, general intelligence, phonological processing skills, expressive and receptive language. We employed a false discovery rate (FDR) of 5% (Benjamini and Hochberg 1995) to control for multiple comparisons across 8 demographic variables and 8 cognitive measures.
Group Comparisons: White Matter Structure
Group differences in FA were examined by calculating, for each tract, a mixed design two-way analysis of variance (ANOVA), with Group (Reader versus Pre-Reader) as a between-subject variable, and Location (30 nodes along the tract) as a within-subject variable (Johnson et al. 2013; Travis, Adams, et al. 2015). Group differences were considered to be significant at p < 0.05 or were considered to demonstrate a significant trend at p < 0.1. To control for multiple comparisons across 13 tracts, we controlled the FDR for the 13 main effects of Group (p<0.05, corrected), and separately controlled the FDR for the 13 Group by Location interaction effects (p<0.05, corrected) (Benjamini and Hochberg 1995). To verify that the data complied with the assumptions of ANOVA, we performed Levene's test for homoscedacity on the mean FA for each tract (Levene 1960). For tracts that were found to have unequal variance, we confirmed the main effect of Group by calculating a one-way ANOVA using Welch's adjustment for unequal variances (Welch 1947). In cases where sphericity was violated, we adjusted the degrees of freedom using Greenhouse-Geisser estimates (Mauchly 1940).
To identify the tract locations (nodes) responsible for group differences identified in the ANOVAs, we followed up on significant Group effects (or Group by location interactions) by calculating two-tailed t-tests for independent samples at each node along the tract profile. We employed a nonparametric permutation-based method to control for the 30 comparisons along the tract (Nichols and Holmes 2002). This procedure produced a family-wise error corrected cluster size and a critical t-value for each of the candidate tracts. Tract segments were considered significant if differences occurred either (1) in a sufficient number of adjacent nodes to meet the criteria for a family-wise error corrected cluster size or (2) in nodes in which the effect size was greater than the critical t-value (Travis, Adams, et al. 2015; Travis, Golden, et al. 2015).
Secondary Analyses: RD, AD
To investigate the contributions of RD and AD to group differences in FA values, we obtained measures for mean RD and mean AD from the cluster of nodes found to demonstrate significant group differences in FA. This analysis was performed only for those tracts found to demonstrate either a significant main effect of Group or a significant interaction effect of Group × Location. We then calculated separate independent samples t-tests for each dependent measure (RD, AD) and each cluster of nodes observed to demonstrate significant group differences in FA. Significance was set at p<0.01 after a rigorous Bonferroni correction.
Secondary Analyses: Associations between FA, Cognitive Measures and Age
To investigate the possible sources of individual variability that may have contributed the observed group differences, we performed a series of correlation analyses. We calculated separate Spearman rank correlations between mean FA from tracts found to demonstrate significant group differences and demographic variables (eg., age) or cognitive measures (eg., phonological awareness, core language, and verbal intelligence) found to differ significantly between groups. We employed Spearman rank correlations due to evidence for non-normal distribution in FA measures. We used an FDR of 5% (Benjamini and Hochberg 1995) to correct for the number of correlations (mean FA: 2 tracts × 4 demographic and cognitive measures = 8 correlations). For exploratory purposes, we also examined these associations with tracts that were found to demonstrate trends for significant group differences. We then performed Spearman partial correlations between FA and cognitive measures, controlling for Word Attack raw scores as continuous variable (used to define group status categorically). These analyses would indicate whether zero-order correlations between FA and cognitive measures could be explained by group differences in both variables (Vandermosten, Boets, Poelmans, et al. 2012). Overall, the purpose of these partial correlations was to determine whether white matter differences could be explained by individual variations in other cognitive abilities not already accounted for by children's reading ability. All correlation analyses were performed excluding the one Pre-Reader subject who was found to be an outlier on phonological awareness (CTOPP) and core language skills (CELF).
Secondary Analyses: Specificity of Group Difference to Reading
To examine whether the observed white matter differences between Reader and Pre-Reader groups reflect structural differences related to reading abilities as opposed to more general cognitive abilities, we repeated group comparisons of the same white matter pathways by dividing the current sample of children into two groups on the basis of their non-verbal intelligence abilities. We used non-verbal IQ as the grouping criterion for this analysis because among the available measures, this one reflected general reasoning ability and was least confounded with reading and associated verbal measures; the correlation between non-verbal IQ and pseudoword reading skills is r = 0.27, p < 0.08 across the full sample. We used a standard score of 110 on the non-verbal IQ test to split the sample into two groups, because this score reflects the empirical population mean for non-verbal intelligence (Waber et al. 2007) and created subgroups of near identical size. Group 1 (High NVIQ) consisted of 22 children who had non-verbal intelligence standard scores of 110 or above and Group 2 (Low NVIQ) consisted of 20 children who had non-verbal intelligence standard scores of below 110. Group differences in FA were examined using the same statistical procedures that were employed to examine group differences between Reader and Pre-Reader groups.
Secondary Analyses: Group Comparisons Controlling for Age
We performed two separate post-hoc analyses to confirm that group differences in FA observed were unlikely to be driven by age. We achieved this first by calculating, for each tract, a mixed design analysis of co-variance (ANCOVA) with Group (Reader versus Pre-Reader) as a between-subject variable, Location (30 nodes along the tract) as a within-subject variable, and age at scan as a covariate (Travis, Adams, et al. 2015). In the second analysis, we individually matched the 11 children in the Pre-Reader group to 11 children in the Reader group on the following criteria (age < 3 months, sex, non-verbal IQ within 1 standard deviation, maternal education, and handedness). For one child in the Pre-reader group the closest age match in the Reader group was 8 months younger. We then compared mean FA from the tract region found to exhibit significant group differences in the full sample of Readers and Pre-Readers using two separate t-tests for paired samples.
Secondary Analyses: Group Comparison of Readers with > 110 Standard Score and Pre-Readers
To ensure that differences in white matter structure were not affected by those children in the Reader group who had achieved only basic pseudoword and real word reading abilities, we repeated group analyses by comparing those children with standardized scores above 110 on both pseudoword and real word tasks to children in the Pre-Reader group. Based on this definition, children in the Reader > 110 standard score (SS) group were able to read a minimum of 5 pseudowords and a minimum of 14 real words. This new analysis included 19 children in the Reader group > 110 standard score (SS) and the same 11 children in the Pre-Reader group. We then repeated group comparisons on demographic, behavioral, and white matter structure variables using the same statistical approaches used to compare the full sample of Readers to Pre-Readers (see above).
Results
Group Comparisons: Demographic and Cognitive Measures
Table 1 summarizes the demographic measures for each group. Children in the Reader group did not differ significantly from those in the Pre-Reader group in sex, ethnicity, SES, stage in school, language background, family history of reading disorders, or handedness (Table 1). The mean age of the Reader group was significantly younger than the mean age of the Pre-Reader group by 2.2 months. Across groups, the percentage of bilingual children were considered to be representative of the demographics of the Bay Area and are consistent with percentages reported for California in a recent survey from the 2011 United States Census (Ryan 2013). One child in the Reader group and one child in the Pre-Reader group were left-handed.
Table 1.
Demographic information for the Reader and Pre-Reader groups.
| Readers (n = 31) M ± SD or n (%) |
Pre-Readers (n = 11) M ± SD or n (%) |
t or X2 | p | |
|---|---|---|---|---|
| Age (years, months) | 6.13y ± 2.0m | 6.31y ± 2.2m | −3.08* | 0.004 |
| Males | 10 (32%) | 5 (46%) | 0.62 | 0.43 |
| White | 20 (65%) | 10 (91%) | 2.77 | 0.10 |
| SES (HI Index) | 58.2 ± 10.2 | 58.1 ± 7.4 | 0.02 | 0.99 |
| Kindergarten | 20 (65%) | 10 (91%) | 2.77 | 0.10 |
| Family History of Reading Problems | 4 (13%) | 4 (36%) | 2.90 | 0.09 |
| Bilingual | 18 (58%) | 3 (27%) | 3.08 | 0.08 |
| Right Handed | 30 (97%) | 10 (91%) | 0.62 | 0.43 |
SES, socioeconomic status; HI, Hollingshead Index.
p < 0.05, corrected
Table 2 summarizes the cognitive measures for each group. By design, children in the Reader group performed significantly higher than children in the Pre-Reader group on pseudoword reading (the defining criterion for group assignment, see Methods). Children in the Reader group also performed significantly higher than children in the Pre-Reader group on single word reading (mean standard score of 120.1 ± 16.9 in the Reader group compared to 86.8 ± 9.3 in the Pre-Reader group).
Table 2.
Standard Scores for Reading, Language, and Cognitive measures in the Reader and Pre-Reader groups.
| Cognitive measure | Reader Mean SS (SD) |
Pre-Reader Mean SS (SD) |
t | p |
|---|---|---|---|---|
| Pseudo-word reading1 | 120.0 (13.0) | 88.3 (5.0) | 7.87 | < 0.001* |
| Real word reading1 | 120.1 (16.9) | 86.8 (9.3) | 6.17 | < 0.001* |
| Phonological Awareness2 | 117.4 (8.7) | 97.6 (12.6) | 5.72 | < 0.001* |
| Phonological Memory2 | 109.4 (15.7) | 102.7 (17.5) | 1.18 | 0.24 |
| Rapid Automatic Naming2 | 97.5 (12.5) | 92.4 (13.1) | 1.15 | 0.26 |
| Core Language3 | 114.0 (13.4) | 100.9 (16.7) | 2.62 | < 0.020* |
| Verbal IQ4 | 126.3 (18.5) | 101.8 (16.2) | 3.87 | < 0.001* |
| Non-Verbal IQ4 | 114.3 (16.8) | 106.3 (12.5) | 1.43 | 0.16 |
p<0.05, corrected
WRMT-III= Woodcock Reading Mastery Tests, 3rd edition
CTOPP = Comprehensive Test of Phonological Processing
CELF 4 = Clinical Evaluation of Language Fundamentals 4th ed.
WASI-II = Weschler Abbreviated Scale of Intelligence 2nd edition
SS = Standardized score
SD = Standard deviation
Children in the Reader group had significantly higher phonological awareness and core language skills than children in the Pre-Reader group (Table 2). Children in the Reader group did not differ significantly from children in the Pre-Reader group on phonological memory or rapid automatic naming skills. Across groups, children had general intelligence levels within the normal to above normal range. Children in the Reader group had significantly higher verbal IQ scores compared to children in the Pre-Reader group. Differences between groups on non-verbal IQ scores did not reach statistical significance.
Group Comparisons: White Matter Structure
Our main analysis compared FA tract profiles in the Reader and Pre-Reader groups along 11 intra-hemispheric tracts (bilateral aSLF, pSLF, UF, CST, ILF and left Arc) and two subdivisions of the corpus callosum (CC-Temporal and CC-Occipital). Mixed design ANOVAs (Group × Location) revealed a significant main effect of group in the aSLF-L (F(1,40)=14.25, p=0.001, corrected) and a trend towards a significant main effect of group in the Arc-L (F(1,39)=3.02, p=0.09). A significant interaction was found between Group and Location in the UF-R (F(3.84,153.46) = 2.75, p=0.03, uncorrected) and a trend towards a significant interaction between Group and Location in the aSLF-L (F(3.9,155.1)=2.46, p=0.05). Table 3 includes the significant and non-significant group differences. One-way ANOVAs using Welch's adjustment confirmed that the significant and non-significant main effect of group for the aSLF-L and UF-R (Table 3) were unlikely to be driven by unequal variance in the aSLF-L (Welch's F(1, 30.96)=23.39, p <0.001) and in the UF-R (Welch's F(1, 30.96)=1.40, p=0.24), respectively. Secondary analyses also confirmed that the main effect of group observed in the aSLF-L remained significant when using the default cleaning parameters (F(1,40) = 10.85, p=0.002).
Table 3.
Results of ANOVA analyses for group comparisons of individual tract FA profiles for Reader and Pre-Reader groups.
| Tract | Reader Mean Tract FA (95% CI) |
Pre-Reader Mean Tract FA (95% CI) |
Main Effect of Group | Group by Location Interaction | ||
|---|---|---|---|---|---|---|
| F | p | F | p | |||
| Arc-L | 0.48 (0.48 - 0.49) | 0.47 (0.45 - 0.49) | 3.02+ | 0.09 | 0.51 | 0.79 |
| aSLF-L | 0.43 (0.41 - 0.44) | 0.38 (0.37 - 0.40) | 14.25** | 0.001 | 2.46+ | 0.05 |
| aSLF-R | 0.46 (0.44 - 0.48) | 0.45 (0.43 - 0.48) | 0.44 | 0.51 | 1.72 | 0.15 |
| pSLF-L | 0.45 (0.44 - 0.46) | 0.43 (0.41 - 0.46) | 1.65 | 0.21 | 0.42 | 0.77 |
| pSLF-R | 0.45 (0.44 - 0.46) | 0.43 (0.41 - 0.46) | 2.78 | 0.10 | 0.38 | 0.79 |
| UF-L | 0.43 (0.42 - 0.44) | 0.43 (0.42 - 0.43) | 0.09 | 0.76 | 1.16 | 0.33 |
| UF-R | 0.44 (0.43 - 0.45) | 0.44 (0.43 - 0.44) | 0.77 | 0.39 | 2.75* | 0.03 |
| CST-L | 0.62 (0.60 - 0.63) | 0.62 (0.60 - 0.64) | 0.07 | 0.77 | 1.00 | 0.41 |
| CST-R | 0.60 (0.59 - 0.62) | 0.61 (0.59 - 0.64) | 0.32 | 0.57 | 0.80 | 0.53 |
| ILF-L | 0.42 (0.41 - 0.43) | 0.42 (0.40 - 0.44) | 0.04 | 0.85 | 1.02 | 0.41 |
| ILF-R | 0.42 (0.41 - 0.43) | 0.42 (0.41 - 0.44) | 0.06 | 0.81 | 0.43 | 0.87 |
| CC-Tempa | 0.71 (0.68 - 0.74) | 0.73 (0.69 - 0.78) | −0.79 | 0.43 | n/a | |
| CC-Occa | 0.72 (0.70 - 0.73) | 0.69 (0.66 - 0.73) | 1.32 | 0.19 | n/a | |
significant p<0.004, corrected
significant p<0.05, uncorrected
trend for significance p<0.1
Mean FA compared with an independent samples t-test
FA = fractional anisotropy; CI = confidence interval; Arc = arcuate fasciculus; aSLF = anterior superior longitudinal fasciculus; pSLF = posterior superior longitudinal fasciculus; UF = uncinate fasciculus; CST = corticospinal tract; ILF = inferior longitudinal fasciculus; CC-Occ = occipital segment of the corpus callosum; CC-Temp = temporal segment of the corpus callosum; L = left; R = right.
We followed up on significant group differences in FA and on significant interactions with a series of two-tailed t-tests comparing mean FA in the Reader and Pre-Reader groups at each node (location) along the tract. The results of these comparisons are visualized in Figure 2. Left-hand panels (Figure 2a, 2c, 2e) show individual tract renderings with a superimposed heat map that depicts the magnitude of two-tailed tests used to identify the location of group differences. Tract locations where two-tailed t-tests were found to be significantly different between Reader and Pre-Reader groups are indicated with colored background shading on FA profiles in right-hand panels (Figure 2b, 2d, 2f). Specifically, light gray shading indicates regions where group differences were significant either at p<0.05 in a sufficient number of adjacent locations to meet the criteria for a family-wise error corrected cluster size or at locations where group differences were greater than the family-wise error corrected critical t-value.
Figure 2.
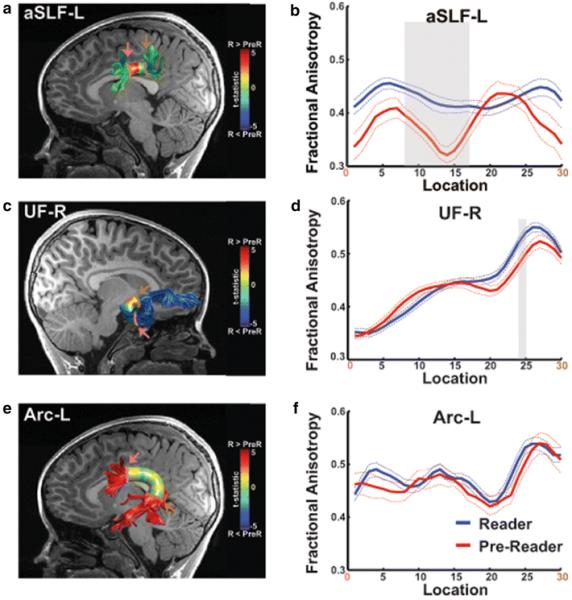
White matter tracts demonstrate FA differences between children in the Reader and Pre-Reader groups. Magnitude of t-tests for independent samples computed to visualize the location of group differences identified in omnibus tests is displayed as a colored heat map on a cylinder surrounding tract renderings for the aSLF-L (a), UF-R (c) and Arc-L (e). Tract renderings are color-coded to match tract renderings presented in Figure 1. FA tract profiles for the Reader (solid blue line) and Pre-Reader (solid red line) groups are shown for aSLF-L (b), UF-R (d) and Arc-L (f). Dashed lines indicate ±1 standard error of the mean. FA values are plotted for 30 equidistant locations (nodes) between the two ROIs used to isolate the core of each tract. Location of ROIs correspond to pink (location 0) and brown (location 30) arrows in FA tract profiles (b,d,f) and T1 images (a,c,e). Shaded gray background indicates tract locations where t-tests for independent samples demonstrated significant group differences using family-wise error correction. aSLF-L = left anterior Superior Longitudinal Fasciculus; UF-R = right uncinate fasciculus; Arc-L = left arcuate fasciculus; R = Reader; PreR = Pre-Reader.
These comparisons revealed that children in the Reader group demonstrated significantly increased FA within a large anterior segment of the aSLF-L tract profile (locations 8-17; Figure 2a,b) compared to children in the Pre-Reader group. Significant differences were also detected in a frontal segment of the UF-R (locations 24-25; Figure 2c,d). A trend for increased FA was also found in Readers compared to Pre-Readers in several distributed locations along of the trajectory of the Arc-L tract profile (Figure 2d,e), however, these comparisons did not reach statistical significance at any single location of the Arc-L.
Secondary Analyses: RD, AD
We next computed t-tests for independent samples to investigate the contributions of RD and AD to group differences in FA observed in the aSLF-L and UF-R in the full sample. In both tracts, RD was significantly decreased in children in the Reader group, compared to children in the Pre-Reader group (aSLF: t= −5.25, p<0.0001, corrected; UF-R: t= −3.01, p=0.006, corrected). No significant group differences were observed in AD for either the aSLF-L (t= 0.26, p=0.80) or UF-R (t=1.83, p=0.08).
Associations between FA, Cognitive Measures and Age
Spearman correlations and partial correlations that were computed to interrogate tract-based group differences are summarized in Table 4 and are visualized as scatter plots in Figure 3. In the entire sample of children, we observed significant positive correlations between mean FA of the aSLF-L and phonological awareness (Figure 3a). Similar findings were found in the Arc-L (Figure 3b). However, after controlling for individual variations in pseudoword reading skills, these correlations with phonological awareness were no longer significant within either the aSLF or Arc-L (Table 4; Figure 3e,f). Thus, the correlation was likely driven by the fact that the Pre-Readers were low on both tract FA and phonological awareness, compared to the Readers.
Table 4.
Spearman correlations and partial correlation between FA and cognitive or demographic measures, with and without controlling for pseudoword reading skills.
| Phonological Awareness | Core Language | Verbal IQ | Age at Scan | |||||
|---|---|---|---|---|---|---|---|---|
| zero-order | partial (WA) | zero-order | partial (WA) | zero-order | partial (WA) | zero-order | partial (WA) | |
| FA | ||||||||
| aSLF-L | 0.39* | 0.05 | 0.34* | 0.18 | 0.29 | -- | −0.22 | -- |
| UF-R | 0.23 | -- | 0.42** | 0.40* | 0.07 | -- | −0.04 | -- |
| Arc-L | 0.37+ | 0.15 | 0.13 | -- | 0.16 | -- | 0.02 | -- |
p < 0.005, corrected
p < 0.05, corrected
p < 0.05, uncorrected
WA = word attack
Figure 3.
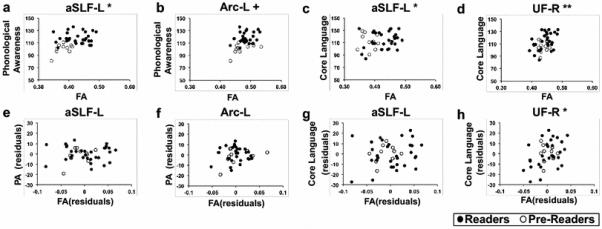
Associations between mean FA from tracts found to exhibit group differences and reading-related cognitive measures before (a-d) and after controlling for individual differences in reading ability (e-h). Partial correlations (e-h) are visualized as a scatter plot between residual FA values and residual values for each cognitive measure. Associations between phonological awareness (PA) and mean fractional anisotropy (FA) of the aSLF-L (a) and Arc-L (b) are significant before, but not after controlling for individual differences in reading (e,f). Associations between core language and mean FA of the aSLF-L (c) are significant before but not after controlling for individual differences in reading (g). Associations between core language and mean FA of the UF-R (d) are significant both before and after controlling for individual differences in reading (h). ** p < 0.005, corrected *p < 0.05, corrected + p < 0.05, uncorrected WA = word attack.
Significant positive correlations across the entire sample were also found between core language skills (CELF) and mean FA of the aSLF-L and the UF-R (Figure 3c,d). Here too, the partial correlation in the aSLF-L was no longer significant after controlling for individual variations in pseudoword reading skills (Figure 3g). However, the partial correlation between FA in the UF-R and core language remained significant after controlling for pseudoword reading (Figure 3h).
No significant correlations were observed between mean FA in the aSLF-L, the UF-R, or the Arc-L and verbal IQ (Table 4). In addition, no significant correlations were observed between mean FA in the aSLF-L, the UF-R, or the Arc-L and age at scanning (Table 4).
Specificity of White Matter Differences to Reading Abilities
To assess whether structural differences observed between Reader and Pre-Reader groups were specific to reading skills or could also be explained by differences in general, non-verbal intelligence, we reran analyses using the full sample of children divided into two groups (High NVIQ and Low NVIQ) and repeated group comparisons of tract FA profiles along the same 11 intrahemispheric tracts as before (bilateral aSLF, pSLF, UF, CST, ILF and left Arc; see Methods). Mixed design ANOVAs (Group × Location) revealed a significant main effect of group was observed within the pSLF-R (F(1,39) = 5.92, p = 0.02, uncorrected; Table 5). No significant main effects of Group or Group × Location effects were observed within any of the tracts found to demonstrate significant group differences between children in the Reader and Pre-Reader groups (aSLF-L, Arc-L, UF-R; Table 5). Table 5 includes the significant and non-significant group differences. A one-way ANOVA using Welch's adjustment confirmed that the non-significant main effect of Group for the Arc-L (Table 5) was unlikely to be driven by unequal variance (Welch's F(1, 29.91)= 0.078, p = 0.78).
Table 5.
Results of ANOVA analyses for group comparisons of individual tract FA profiles for children with high non-verbal IQ standard scores (Group 1) and children with low non-verbal IQ standard scores (Group 2).
| Tract | Main Effect of Group | Group by Location Interaction | ||
|---|---|---|---|---|
| F | p | F | p | |
| Arc-L | 0.808 | 0.78 | 1.44 | 0.21 |
| aSLF-L | 0.15 | 0.70 | 1.53 | 0.20 |
| aSLF-R | 1.33 | 0.26 | 1.21 | 0.31 |
| pSLF-L | 2.87 | 0.10 | 0.99 | 0.41 |
| pSLF-R | 5.92* | 0.02 | 0.45 | 0.74 |
| UF-L | 0.72 | 0.40 | 0.12 | 0.98 |
| UF-R | 0.00 | 0.97 | 0.15 | 0.95 |
| CST-L | 0.59 | 0.45 | 0.96 | 0.42 |
| CST-R | 0.09 | 0.76 | 0.48 | 0.76 |
| ILF-L | 0.07 | 0.79 | 1.59 | 0.15 |
| ILF-R | 1.27 | 0.27 | 0.83 | 0.56 |
| CC-Tempa | −1.01 | 0.32 | n/a | |
| CC-Occa | 0.56 | 0.58 | n/a | |
significant p<0.05, uncorrected
+ trend for significance p<0.1
Mean FA compared with an independent samples t-test
FA = fractional anisotropy; CI = confidence interval; Arc = arcuate fasciculus; aSLF = anterior superior longitudinal fasciculus; pSLF = posterior superior longitudinal fasciculus; UF = uncinate fasciculus; CST = corticospinal tract; ILF = inferior longitudinal fasciculus; CC-Occ = occipital segment of the corpus callosum; CC-Temp = temporal segment of the corpus callosum; L = left; R = right.
Group Comparisons Controlling for Age
Two separate post-hoc analyses confirmed that group differences in FA observed for the aSLF-L and UF-R were unlikely to be driven by children in the Reader group being significantly younger (by ~2 months) than children in the Pre-Reader group. Specifically, ANCOVA (Group × Location) analyses revealed that the aSLF-L continued to demonstrate a significant main effect of group (F(1, 40)=12.57, p=0.001; Table S1) and a significant interaction effect (F(3.87,150.77)=2.78, p=0.03; Table S1) after controlling for age. ANCOVA (Group × Location) analyses also revealed that the UF-R demonstrated a trend for a significant interaction between Group and Location (F(3.80,148.37)=2.50, p=0.05; Table S1) after controlling for age. Neither tract demonstrated significant main effect of age (Table S1). In addition, t-tests for paired samples confirmed that a matched sample of 11 Readers continued demonstrate significantly increased FA than the 11 children in the Pre-Reader group within the aSLF-L (tract locations 8-17; t = 3.09, p = 0.01) and UF-R (tract locations: 24-25; t = 3.36, p = 0.007).
Group Comparison of Readers with > 110 Standard Score and Pre-Readers
Similar to findings in the full sample of Readers, we found no significant group differences between Readers > 110 SS and Pre-Readers on the basis of sex, race, socio-economic status (SES), stage in school, family history of reading problems, bilingualism, or handedness p > 0.05 (Table S2). Children in the Pre-Reader group remained significantly older than children in the Reader > 110 SS group, however this difference was no longer significant after controlling for the number of comparisons (Table S2). Group comparisons repeated for behavioral variables revealed the same group differences as observed for the full sample of Readers (Table S3).
Group comparisons of white matter structure revealed a similar pattern of group differences as observed in the full sample of Readers. Mixed design ANOVAs (Group × Location) revealed a significant main effect of group in the aSLF-L (F(1,28) = 11.56, p = 0.002, corrected; Table S4) and a significant interaction was found between Group and Location in the UF-R (F(4.09, 114.60)=2.98, p=0.02, uncorrected; Table S4). Children in the Reader > 110 SS group no longer demonstrated a trend for a main effect of group within the Arc-L or a group by location effect within the aSLF-L. We attribute this negative finding to reduced power from removing 12 subjects from the Reader group. One-way ANOVAs using Welch's adjustment confirmed that the significant main effect of Group for the aSLF-L (Table S4) was unlikely to be driven by unequal variance in the aSLF-L (Welch's F(1, 28)=15.40, p=0.001).
Discussion
The present study provides novel evidence that children who are able to read demonstrate structural white matter differences compared to children who are not able to read. Specifically, we found in a sample of healthy 6-year old children that children classified as Readers had significantly increased FA in segments of the aSLF-L and the UF-R, and a trend for increased FA within the Arc-L compared to children classified as Pre-Readers. Increased FA within the aSLF-L and UF-R was driven by decreased RD. We concluded that group differences were not due to sex, socio-economic status, amount of schooling, and family history for reading disorders because the groups did not differ on these demographic variables. The mean age of the Readers was 2-months younger than the mean age of the Pre-Readers, but age was not associated with any of the group differences in FA. We found positive correlations between FA in the aSLF-L and Arc-L and phonological awareness. Partial correlations revealed that these associations could be explained by group differences in both variables (FA and phonological awareness). We also found positive associations between FA and core language in the aSLF-L and UF-R. Partial correlations revealed that the association in the aSLF-L was also explained by group differences in both variables (FA and core language). However, group differences in FA in the UF-R could be explained by individual variations in children's core language skills in addition to their reading abilities. Post-hoc group analyses further confirmed that group differences in the aSLF-L, UF-R and were likely to reflect anatomical differences related to reading abilities as opposed to general non-verbal cognitive abilities. Additional post-hoc analyses also confirmed that group differences observed for the aSLF-L and UF-R were unlikely to be affected by group differences in age and by those children in the Reader group who had achieved only basic pseudoword and real word reading abilities. Taken together, these findings show that differences in white matter connectivity in the earliest stages of reading education relate to whether children have acquired specific reading skills.
Consistent with our initial predictions, we observed significant group differences within left dorsal white matter pathways that have previously been implicated in reading (Deheane 2009; Vandermosten, Boets, Wouters, et al. 2012; Wandell and Yeatman 2013). The most significant FA differences were found within fibers of the anterior subdivision of the left SLF. The aSLF has been directly implicated in articulatory processes based on findings from inter-operative mapping studies showing that stimulation of this tract produces speech arrest (Duffau et al. 2002; Duffau et al. 2003; Duffau 2008). Children who are just learning to read typically read out loud and may thus rely heavily on dorsal pathways such as the left aSLF to access phonological and articulatory processing information. Longitudinal studies will be important for determining whether differences observed in the aSLF-L disappear as children become more proficient at reading and are able to recognize letter strings and access word meaning automatically. Such studies will be important for establishing whether the relative involvement of white matter tracts in reading changes over reading development.
We found a trend for group differences in the Arc-L. Evidence for reading-based differences in this pathway in conjunction with the finding that mean FA of the Arc-L was associated with phonological awareness skills is consistent with numerous developmental dMRI studies implicating the left arcuate in reading and phonological skills (Yeatman et al. 2011; Vandermosten, Boets, Wouters, et al. 2012; Saygin et al. 2013; Gullick and Booth 2014; Vanderauwera et al. 2015). We determined, by performing partial correlations controlling for reading ability, that this association was primarily a reflection of group differences between Readers and Pre-Readers in two separate measures: (a) differences in mean FA of the Arc-L; and (b) differences in phonological awareness skills. This pattern of correlations was also found for the aSLF-L. These findings suggest that white matter differences observed for the Arc-L and aSLF-L likely reflect differences in the acquisition of cognitive abilities relevant for both decoding and phonological skills. Further studies employing cognitive tasks more specific than those employed here might be important for specifying the functional roles of the aSLF-L and Arc-L in reading-related processes. Overall, these patterns of findings are generally consistent with cognitive models of language and reading that consider dorsal white matter pathways key for phonological processes relevant for mapping auditory to motor information (Ben-Shachar et al. 2007; Deheane 2009; Price 2012; Vandermosten, Boets, Wouters, et al. 2012; Wandell and Yeatman 2013).
Our findings are generally compatible with results from a recent dMRI study that found differences between adults who learned to read (either as children or adults) compared to adults who had not learned to read (Thiebaut de Schotten et al. 2014). In that study, group differences were confined to the pSLF, whereas we found differences in the aSLF-L and a trend for group differences in the Arc-L. Variations in the location of group differences may relate to differences in analytic methods. It is important to note that fibers belonging to different subdivisions of the SLF (aSLF, arcuate, pSLF) are located in close proximity to one another and are likely to share voxels in some regions (Tsang et al. 2009). The overlap may occur frequently in temporal regions where fibers of the left arcuate may share voxels with the pSLF and in frontal regions where fibers of the left arcuate may share voxels with the aSLF. The observed findings seen here and elsewhere in adults may thus reflect a more general pattern of involvement of the left dorsal pathways in the acquisition of reading skills. Future dMRI studies involving larger samples of both children and adults are likely to be important for specifying the role for distinct subdivisions of the left SLF and arcuate in learning to read.
The present study presents novel evidence that the early acquisition of reading may also involve fibers that extend beyond classic left dorsal and ventral pathways for reading, including those of the right uncinate. Because we employed an analytic approach that accounted for along tract variations in FA, we were able to observe group differences that may have otherwise been obscured with mean tract measures. Moreover, we found that individual variations in FA of the right uncinate were associated with individual differences in children's expressive and receptive language skills. Behavioral studies find that individual differences in reading are associated with individual differences in language abilities, particularly at the early stages of reading (Catts et al. 1999; Storch and Whitehurst 2002; NICHD Early Child Care Research Network 2005). While there remains considerable disagreement as to the exact functions of the uncinate fasciculus, the present associations are consistent with evidence implicating the uncinate in language-related functions such as auditory working memory (McDonald et al. 2008; Papagno 2011; Dick and Tremblay 2012). Moreover, the present findings are also compatible with evidence implicating the uncinate in language processes relevant for reading, including lexical and semantic retrieval (Von Der Heide et al. 2013). These findings are also consistent with two separate developmental dMRI studies of reading that observed positive associations between FA of the right uncinate and real and pseudoword reading skills in children and adolescents born preterm (Feldman et al. 2012) and in a sample of typically developing children and children with dyslexia (Odegard et al. 2009). Similar associations between phonemic decoding abilities and FA have also been reported in adults, however these associations were primarily observed in the left uncinate fasciculus (Welcome and Joanisse 2014; Cummine et al. 2015). Understanding how both the left and right uncinate contribute to reading is likely to benefit from research that combines both structural and functional neuroimaging techniques.
By comparing children on the basis of their non-verbal intelligence abilities we were able to show that the group differences observed between Readers and Pre-Readers are unlikely to reflect differences in general cognitive skills. Similarly, verbal intelligence measures, which were only modestly associated with reading skills in the current sample, were not associated with mean FA from any of the tracts found to demonstrate reading-related group differences. On the other hand, correlation analyses across the entire sample detected significant association between phonological awareness scores and mean tract FA of the aSLF-L and Arc-L. Indeed, phonological awareness skills are strongly correlated with pseudoword reading skills in our sample (r=0.76, p<0.0001), and are known to be highly related to reading abilities in very young readers (Castles and Coltheart 2004; Melby-Lervag et al. 2012). Thus, the group differences we describe between Readers and Pre-Readers may not be disentangled from, and may well be driven by, differences in phonological awareness, a critical skill involved in early literacy acquisition.
We suggest that increased FA observed here for children in the Reader group is likely to reflect enhancements in cellular properties that afford efficient signal transmission. Higher FA has typically been associated with favorable neurobiological factors, such as increased myelination and greater axonal count (Basser and Pierpaoli 1996; Beaulieu 2002). There is also evidence from animal studies that reductions in RD are associated with increases in myelination (Song et al. 2002; Song et al. 2005). Here, the finding for increased FA in combination with reduced RD, suggests that children who are able to read may have increases in myelination, axonal densities, or fiber coherence relative to children who are not able to read. However, dMRI methods by themselves cannot distinguish amongst these possibilities (Jones and Cercignani 2010; De Santis et al. 2014). Understanding the neurobiological properties contributing to these group differences will benefit from the combination of diffusion measures with other quantitative MRI methods for estimating myelin content or axonal diameter more directly (Assaf et al. 2008; Mezer et al. 2013; Tardif et al. 2015; Travis, Golden, et al. 2015).
The present study cannot determine whether the observed white matter differences reflect cellular enhancements resulting from experience with reading, experience with pre-reading activities such as language learning, or pre-existing genetic traits that facilitate initial acquisition of reading. Deciphering amongst these possibilities cannot be achieved in observational and correlational studies, and is instead likely to require studies that are designed to directly manipulate the learning environment of the developing child (Keller and Just 2009; Webb et al. 2015). Nevertheless, the present findings have important implications for prior developmental studies of pre-readers and early readers that have observed white matter differences in the context of identifying predictive biomarkers of reading impairments in samples of children with specific behavioral or familial risk factors for reading disorders (Saygin et al. 2013; Myers et al. 2014; Langer et al. 2015; Vanderauwera et al. 2015; Vandermosten et al. 2015). Our findings show that differences in white matter structure can also be explained by whether children have acquired specific abilities for reading. Taken together, such findings underscore the likelihood that white matter differences in children are likely to be influenced by both experiential or environmental and genetic factors.
A limitation of the present study was that our sample size of pre-readers was small. However, our sample size is directly comparable to the one in (Thiebaut de Schotten et al. 2014). Because we could not obtain reliable tracking of the Arc-R in 2 children in the Pre-Reader group, the present sample limited our ability to determine whether group differences existed between Reader and Pre-Reader groups in this pathway. Establishing whether children who are able to read demonstrate structural differences within the Arc-R will likely require the use of probabilistic tractography approaches, which have been used to successfully identify the Arc-R in individuals in whom deterministic approaches have failed (Yeatman et al. 2011). Another potential limitation of the present study was that our sample of Readers was found to be 2 months younger than children in the Pre-reader group. While it is possible that the observed group differences may be related to group differences in age, this seems unlikely for several reasons. Firstly, the age difference was in the unexpected direction, with children in the Reader group being younger, as opposed to older, than children in the Pre-Reader group. Had children in the Reader been significantly older, there would have been more concern that children in the Reader group were able read because of increased experience with reading and that the observed white differences may have been the result of developmental increases in FA (Lebel and Beaulieu 2011; Lebel et al. 2012). Secondly, we demonstrated in two separate follow-up analyses that group differences persisted after controlling for age (see Table S1). Thirdly, we demonstrated that age was not significantly related to FA in tract regions found to demonstrate group differences in FA (see Table 4). A final limitation of the present study was that we cannot determine whether children in the Reader group were able to read because of environmental factors that provided them with experiences necessary for learning or whether these children have pre-existing traits that enabled the to read. In future studies, we will be able to establish whether the observed structural differences disappear following the acquisition of reading or persist, suggestive of a neurobiological basis of differences in learning abilities.
In summary, the present findings provide new evidence showing that children who are able to read have white matter differences as compared to similarly aged children who cannot read. The location of group differences suggests that the acquisition of reading may involve both left and right hemisphere pathways. The present findings underscore that future developmental studies should consider whether children have acquired specific cognitive abilities required for reading when interpreting white matter differences across samples of differing reading abilities or risk status. Future studies employing quantitative MRI methods for measuring axonal caliber and myelin will be important for distinguishing amongst the possible neurobiological factors underlying the observed group differences. Overall, the present findings demonstrate the potential use of dMRI and tractography in helping to understand how the acquisition of specific cognitive abilities impacts white matter development.
Supplementary Material
Acknowledgements
We thank the children and families who participated in our study.
Funding
This work was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development at the National Institutes of Health (grant numbers RO1-HD69162, RO1-HD46500); the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant number 51/11) to MB-S.
References
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. NeuroImage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Stastistical Society. 1995;57:289–300. [Google Scholar]
- Brauer J, Anwander A, Perani D, Friederici AD. Dorsal and ventral pathways in language development. Brain Lang. 2013;127:289–295. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Castles A, Coltheart M. Is there a causal link from phonological awareness to success in learning to read? Cognition. 2004;91:77–111. doi: 10.1016/s0010-0277(03)00164-1. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Miranda PC, Carmo I, Reis A, Leote F, Ribeiro C, Ducla-Soares E. Influence of learning to read and write on the morphology of the corups callosum. Eur J Neurol. 1999;6:23–28. doi: 10.1046/j.1468-1331.1999.610023.x. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catts HW, Fey ME, Zhang X, Tomblin JB. Language basis of reading and reading disabilities: evidence from a longitudinal investigation. Scientific Studies of Reading. 1999;3:331–361. [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med. 2005;53:1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummine J, Dai W, Borowsky R, Gould L, Rollans C, Boliek C. Investigating the ventral-lexical, dorsal-sublexical model of basic reading processes using diffusion tensor imaging. Brain structure & function. 2015;220:445–455. doi: 10.1007/s00429-013-0666-8. [DOI] [PubMed] [Google Scholar]
- De Santis S, Drakesmith M, Bells S, Assaf Y, Jones DK. Why diffusion tensor MRI does well only some of the time: Variance and covariance of white matter tissue microstructure attributes in the living human brain. NeuroImage. 2014;89:35–44. doi: 10.1016/j.neuroimage.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deheane S. Reading in the Brain: The Science and Evolution of Human Invention. Viking Penguin; New York: 2009. [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT. Children's Reading Performance is Correlated with White Matter Structure Measured by Diffusion Tensor Imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Bammer R, Brewer AA, Wandell BA. Functional organization of human occipital-callosal fiber tracts. Proc Natl Acad Sci U S A. 2005;102:7350–7355. doi: 10.1073/pnas.0500003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academy of Sciences. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez J-P, Bitar A, Fohanno D. Intraoperative mapping of the subcortical language pathways using direct stimulations An anatomo-functional study. Brain. 2002;125:199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Denvil D, Lopes M, Capelle L. The articulatory loop: study of the subcortical connectivity by electrostimulation. Neuroreport. 2003;14:2005–2008. doi: 10.1097/00001756-200310270-00026. [DOI] [PubMed] [Google Scholar]
- Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50:3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. Neuroimage. 2004;23:21–24. doi: 10.1016/j.neuroimage.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Liederman J, Papanicolaou A. Splenium microstructure is related to two dimensions of reading skill. Neuroreport. 2008;19:1627–1631. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PB, Tunmer WE. Decoding, reading, and reading disability. Remedial Special Education. 1986;7:6–10. [Google Scholar]
- Groeschel S, Tournier JD, Northam GB, Baldeweg T, Wyatt J, Vollmer B, Connelly A. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage. 2014;87:209–219. doi: 10.1016/j.neuroimage.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Gullick MM, Booth JR. Individual differences in crossmodal brain activity predict arcuate fasciculus connectivity in developing readers. J Cogn Neurosci. 2014;26:1331–1346. doi: 10.1162/jocn_a_00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick MM, Booth JR. The direct segment of the arcuate fasciculus is predictive of longitudinal reading change. Dev Cogn Neurosci. 2015;13:68–74. doi: 10.1016/j.dcn.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover GH, Keller TA, Kobayashi N, Mazaika P, Jo B, Just MA, Gabrieli JDE. Prediction of children's reading skills using behavioral, functional, and structural neuroimaging measures. Behavioral Neuroscience. 2007;121:602. doi: 10.1037/0735-7044.121.3.602. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Yeatman JD, Wandell BA, Buonocore MH, Amaral DG, Nordahl CW. Diffusion properties of major white matter tracts in young, typically developing children. NeuroImage. 2013;88:143–154. doi: 10.1016/j.neuroimage.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JR, Desrochers A, Roth L, Lai S. Longitudinal predictors of word reading development. Canadian Psychology/ Psychologie canadienne. 2008;49:103–110. [Google Scholar]
- Langer N, Peysakhovich B, Zuk J, Drottar M, Sliva DD, Smith S, Becker BL, Grant PE, Gaab N. White Matter Alterations in Infants at Risk for Developmental Dyslexia. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30:3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Levene H. Robust tests for equality of variances. Stanford University Press; Stanford, California: 1960. [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain structure & function. 2013;218:105–121. doi: 10.1007/s00429-012-0386-5. [DOI] [PubMed] [Google Scholar]
- Mauchly JW. Significance test for sphericity of a normal n-variate distribution. The Annals of Mathematical Statistics. 1940;11:204–209. [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby-Lervag M, Lyster S-AH, Hulme C. Phonological Skills and Their Role in Learning to Read: A Meta-Analytic Review. Psychological Bulletin. 2012;138:322–352. doi: 10.1037/a0026744. [DOI] [PubMed] [Google Scholar]
- Mezer A, Yeatman JD, Stikov N, Kay KN, Cho NJ, Dougherty RF, Perry ML, Parvizi J, Hua le H, Butts-Pauly K, Wandell BA. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med. 2013;19:1667–1672. doi: 10.1038/nm.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Anderson AW, Wu X, Gore JC, Ding Z. An improved Bayesian tensor regularization and sampling algorithm to track neuronal fiber pathways in the language circuit. Med Phys. 2010;37:4274–4287. doi: 10.1118/1.3456113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzalvo K, Dehaene-Lambertz G. How reading acquisition changes children's spoken language network. Brain Lang. 2013;127:356–365. doi: 10.1016/j.bandl.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko V, Van Zijl P. Three - dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, Casto B, Drahos M, Tumber M, Hendren RL, Hulme C, Hoeft F. White Matter Morphometric Changes Uniquely Predict Children's Reading Acquisition. Psychological Science. 2014;25:1870–1883. doi: 10.1177/0956797614544511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cognitive Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network Pathways to reading: the role of oral language in the transition to reading. Dev Psychol. 2005;41:428–442. doi: 10.1037/0012-1649.41.2.428. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Current neurology and neuroscience reports. 2011;11:553–559. doi: 10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Press W, Teukolsky S, Vetterling W, Flannery B. Numerical Recipes in C++:The Art of Scientific Computing. Cambridge Univ Press; Cambridge, UK: 2002. [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41:223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46:739–749. doi: 10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51:103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Ryan C. Language Use in the United States: 2011. American Community Survey Reports. 2013 [Google Scholar]
- Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Palchik O, Yendiki A, Fischl B, Gaab N, Gabrieli JDE. Tracking the Roots of Reading Ability: White Matter Volume and Integrity Correlate with Phonological Awareness in Prereading and Early-Reading Kindergarten Children. The Journal of neuroscience. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Share DL. Phonological recoding and self-teaching: sine qua non of reading acquisition. Cognition. 1995;55:151–218. doi: 10.1016/0010-0277(94)00645-2. [DOI] [PubMed] [Google Scholar]
- Snowling M, Dawes P, Nash H, Hulme C. Validity of a protocol for adult self-report of dyslexia and related difficulties. Dyslexia. 2012;18:1–15. doi: 10.1002/dys.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Storch SA, Whitehurst GJ. Oral language and code-related precursors to reading: Evidence from a longitudinal structural model. Dev Psychol. 2002;38:934–947. [PubMed] [Google Scholar]
- Tardif CL, Gauthier CJ, Steele CJ, Bazin PL, Schafer A, Schaefer A, Turner R, Villringer A. Advanced MRI techniques to improve our understanding of experience-induced neuroplasticity. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.08.047. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 2014;24:989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Travis KE, Adams JN, Ben-Shachar M, Feldman HM. Decreased and Increased Anisotropy along Major Cerebral White Matter Tracts in Preterm Children and Adolescents. PLoS One. 2015;10:e0142860. doi: 10.1371/journal.pone.0142860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Golden NH, Feldman HM, Solomon M, Nguyen J, Mezer A, Yeatman JD, Dougherty RF. Abnormal white matter properties in adolescent girls with anorexia nervosa. NeuroImage: Clinical. 2015;9:648–659. doi: 10.1016/j.nicl.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JM, Dougherty RF, Deutsch GK, Wandell BA, Ben-Shachar M. Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. Proc Natl Acad Sci U S A. 2009;106:22546–22551. doi: 10.1073/pnas.0906094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera J, Vandermosten M, Dell'Acqua F, Wouters J, Ghesquiere P. Disentangling the relation between left temporoparietal white matter and reading: A spherical deconvolution tractography study. Hum Brain Mapp. 2015;36:3273–3287. doi: 10.1002/hbm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquiere P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, Wouters J, Ghesquiere P. A DTI tractography study in pre-readers at risk for dyslexia. Dev Cogn Neurosci. 2015;14:8–15. doi: 10.1016/j.dcn.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Paus T, Rumsey J, Brain Development Cooperative G. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen J, Rashotte C. Comprehensive test of phonological processing: CTOPP: Pro-Ed. 1999 [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Yeatman JD. Biological development of reading circuits. Curr Opin Neurobiol. 2013;23:261–268. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AR, Heller HT, Benson CB, Lahav A. Mother's voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci U S A. 2015;112:3152–3157. doi: 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Hsiao-pin C. WASI-II: Wechsler abbreviated scale of intelligence: Pearson. 2011 [Google Scholar]
- Welch BL. The generalization of Student's problem when several differenct population variances are invovled. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- Welcome SE, Joanisse MF. Individual differences in white matter anatomy predict dissociable components of reading skill in adults. Neuroimage. 2014;96:261–275. doi: 10.1016/j.neuroimage.2014.03.069. [DOI] [PubMed] [Google Scholar]
- Wigg E, Secord W, Semel E. Clinical evaluation of langauge fundamentals (CELF-4) Hartcourt Psychology Corporation Assessments, Inc.; San Antonio, TX: 2003. [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Test. Third Edition. Pearson; San Antonio: 2011. [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc Natl Acad Sci U S A. 2012;109:E3045–3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS ONE. 2012;7:e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cognitive Neurosci. 2011;23:3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nature communications. 2014;5:4932. doi: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


