Abstract
A woman aged 66 years with a history of unprovoked deep venous thrombosis (DVT) presented with persistent digital ischaemic changes of 2 of her right hand fingers. Physical examination was otherwise normal and extensive laboratory and imaging studies were unremarkable. A history of unprovoked DVT and the current episode of digital ischaemia prompted concern for underlying occult malignancy. Repeated history-taking revealed a strongly positive family history suggesting an occult colorectal cancer. Colonoscopy with biopsy revealed adenocarcinoma. Adenocarcinoma of the colon has rarely been associated with paraneoplastic acral vascular syndrome. This report suggests that occult malignancy needs to be considered in patients with focal digital ischaemia as this association is poorly unrecognised.
Background
Various dermatological clues may occur as the presenting manifestation of an occult cancer. These so-called paraneoplastic dermatoses do not involve infiltration of the skin by malignant cells.1 The acquired (non-inherited) entities include hyperkeratotic and proliferative dermatoses (eg, acanthosis nigricans, acquired ichthyosis); Inflammatory dermatoses (eg, Sweet syndrome, dermatomyositis, panniculitis); bullous diseases (eg, paraneoplastic pemphigus); non-thrombocytopenic purpura/petechiae (eg, cutaneous vasculitis, amyloidosis); scleroderma-like dermatoses; manifestations of hormone-secreting tumours (eg, carcinoid syndrome, Cushing syndrome) and pruritus. We report a rare, poorly recognised association of focal small-vessel digital ischaemia representing paraneoplastic acral vascular syndrome2 and occult colon cancer.
Case presentation
A woman aged 66 years was admitted with a 2-week history of a gradual change in appearance (cyanotic mottling), temperature (coolness) and feeling (dysaesthesiae, pain) limited to two fingers (3rd and 4th fingers) of her right hand (figure 1). The changes were worse in the morning and on exposure to cold weather, improved on warming or massage but remained constantly present. All pulses were palpated and no ulcerations were found.
Figure 1.
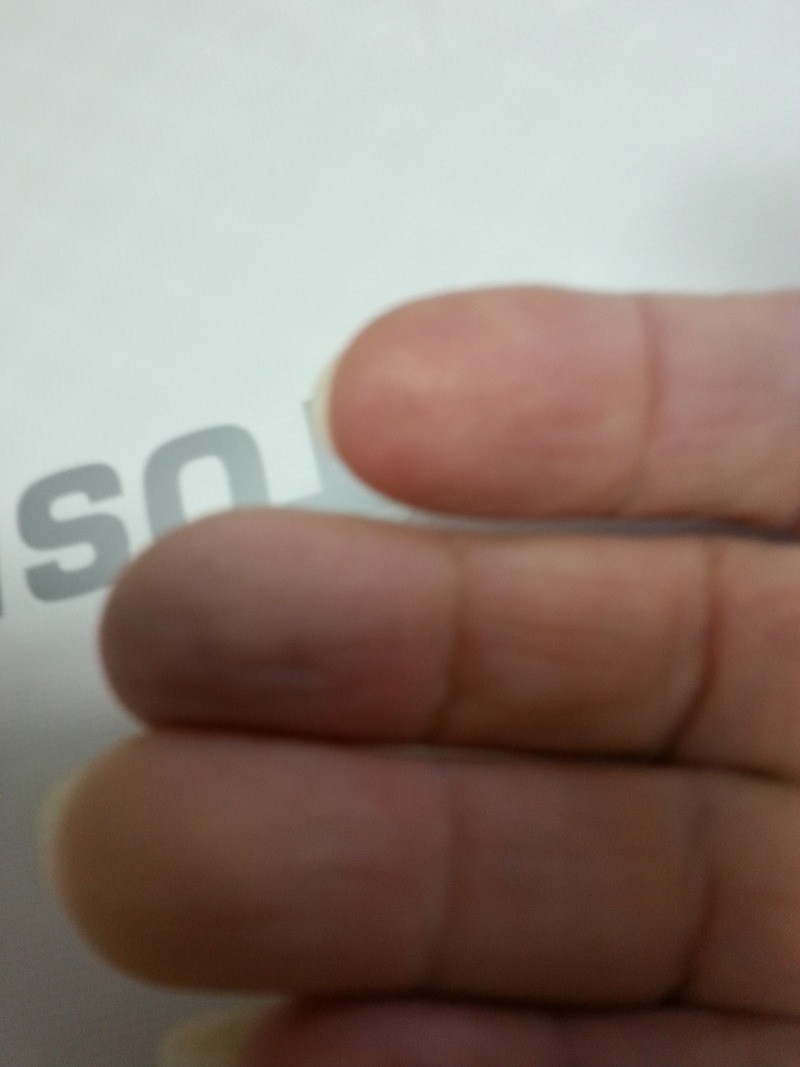
The patient’s right hand fingers on admission showing cyanotic mottling of two fingers, characteristic of paraneoplastic acral vascular syndrome. Peripheral pulses were all normal.
The patient had never smoked and had always been healthy except for one episode of unprovoked iliofemoral deep vein thrombosis (u-DVT) 12 months prior to her current presentation with digital ischaemic changes. She was neither hospitalised nor evaluated and had received warfarin for 6 months. On admission, review of systems was unremarkable. She was taking no medications. The family history was inadvertently omitted on admission. The physical examination was normal, except for the two involved fingers.
Investigations
Chest X-ray, ECG, a comprehensive laboratory workup and abdominal ultrasound were normal. The Hb was 13.2 g/dL (MCV 86), WCC 8.74×103/µL, platelets 169×103/µL. Acute-phase reactants were not increased and no autoantibodies were found. A workup for antiphospholipid syndrome (APS) was negative, including lupus anticoagulant, anticardiolipin and anti-β2 glycoprotein antibodies. VDRL was negative. Serum protein immunoelectrophoresis was normal. Neither cryoglobulins or cryofibrinogen nor cold agglutinins were found. JAK2 mutation was not found. Echocardiography (transthoracic and transoesophageal) was normal: neither an embolic source (such as cardiac vegetations or tumour), nor an atrial septal defect or patent foramen ovale (that could be associated with paradoxical emboli to the fingers) was found. Contrast-enhanced chest, abdominal and pelvic CT and mammography showed no malignancy or other pathological findings. The colon appeared normal. Low-molecular weight heparin (LMWH) treatment was started, and her fingers improved after several days. She was about to be discharged for ambulatory follow-up when we found after noticing that family history was missing from her admission notes that her mother and aunt had been diagnosed with colorectal cancer (CRC). She herself had been under surveillance for no less than 24 years, but did not mention it. Her last colonoscopy had been performed 5 years previously and revealed only melanosis coli, a benign finding. Despite the absence of symptoms and normal blood count, faecal occult blood testing (FOBT) was now strongly positive (6/6 samples). Colonoscopy demonstrated a circular ulcerated sigmoid adenocarcinoma 20 cm from the anus. It was found to be 2.7 cm in diameter, a reminder of the poor sensitivity of CT scanning (but not CT colonography) for the detection of colon cancer, and sigmoid cancer in particular.
Differential diagnosis
In addition to the entities that will be discussed in more detail below, we considered the possibility of medication-induced vasoconstriction (eg, amphetamines), occlusive vascular disease (eg, thrombangiitis obliterans), vascular compression (eg, thoracic outlet syndrome), vasculitis (eg, Behcet’s disease), carpal tunnel syndrome and complex regional pain syndrome. Each of these could be ruled out on clinical grounds. Paradoxical embolism from a recurrent silent DVT was also considered and ruled out.
Treatment
The colon cancer was resected revealing 5/18 positive lymph nodes (stage T3N2M0). The patient was started on chemotherapy and continued LMWH treatment at a daily dose of 1.5 mg/kg.
Outcome and follow-up
The digital ischaemia improved and did not recur. This is consistent with the literature where 48% of the cases showed improvement after treatment of the underlying tumour.2 On oncological follow-up, there is no evidence of disease recurrence at 2 years.
Discussion
In similarity with the ‘blue toe syndrome’,3 our patient presented with symptoms and skin changes strongly suggesting partial, small-vessel digital ischaemia with preserved pulses. The insightful triad ascribed to Rudolf Virchow (Berlin, mid-19th century)4 can be applied here, stating that sluggish flow, vessel wall damage or abnormal blood constituents may underlie ischaemic fingers. Thus, the differential is broad including cardiogenic or aortic embolism and atheroembolism, vessel wall inflammation or constriction and abnormal blood composition including paraproteinaemia, cold agglutinins, cryofibrinogenaemia, cryoglobulinaemia or changes associated with two myeloproliferative disorders (MPD)—polycythaemia vera or essential thrombocythaemia.2 Primary Raynaud's phenomenon affects ∼10% of the population,5 but unlike our patient usually appears at a younger age (and usually not 66 years of age); and is bilateral (and not unilateral); paroxysmal, episodic and reversible (and not persistent); sudden (and not gradual); and typically shows colour changes from white to blue and red (and not a steady bluish discolouration). In contrast, secondary Raynaud's phenomenon is diagnosed in ∼1:10 patients.6 It tends to develop at older ages, and may be asymmetric and more severe with more pain and ulcerations or gangrene. Many of these patients have autoimmune rheumatic diseases or haematological abnormalities.7 A paraneoplastic Raynaud's phenomenon—the so-called ‘paraneoplastic acral vascular syndrome’—is a distinctly rare occurrence.2 3 8
However, our patient also had a recent unprovoked DVT and Occam’s principle of parsimony9 urges us to first consider a single explanation, one diagnosis responsible for both events. Few entities can cause both venous (u-DVT) and arterial hypercoagulability. When APS, MPD and Behcet’s disease were ruled out, malignancy remained the prime suspect. However, extensive tests and imaging failed to discover the suspected malignancy, until the patient’s history—that simplest, safest and cheapest of all tests—provided the missing clue and led to the diagnosis of CRC. In our patient, the family history of CRC was almost forgotten. Unfortunately, this omission is not uncommon.10
Indeed, occult cancer can be detected in a significant number of patients >40 years who experience an unprovoked DVT and investigations are often recommended, although their nature, survival benefit and cost-effectiveness remain controversial.11 Adenocarcinoma are a major cause of cancer-associated unprovoked DVT, predominantly by the release of procoagulant tissue factor-bearing microvesicles from mucin-producing tumour cells.12
However, a variety of other mechanisms of digital ischaemia in malignancy had been identified including emboli from non-bacterial thrombotic endocarditis,13 hyperviscosity of paraproteinaemia, MPD, cryoglobulinaemia, cryofibrinogenaemia, cold agglutinins and possible release of vasoconstrictor substances by the tumour.2
A literature review that analysed 68 patients with paraneoplastic acral vascular syndrome found a median age of 59 years; a persistent rather than paroxysmal occurrence with a high percentage of ischaemic complications such as necrosis and gangrene (40/68, 59%); a predominance of adenocarcinoma (41%) and common regression after treatment of the tumour.2 In a subsequent cohort study of 100 patients with a first episode of digital ischaemia, only 12 (12%) were diagnosed with cancer in the year preceding or following, and only 5 had adenocarcinoma.14 Raynaud's phenomenon (often unilateral) may precede digital ischaemia. In our patient, colon cancer proved an extremely rare and poorly recognised cause of digital ischaemia, previously reported in only three patients.14–16 In addition, we were not able to find previous reports of patients whose presentation of adenocarcinoma included hypercoagulability manifestations of the arterial (finger ischaemia) and venous (DVT) circulations.
Learning points.
When venous and arterial manifestations of hypercoagulability occur in the same patient (such as unprovoked deep vein thrombosis and digital ischaemia, respectively), the differential is quite narrow and an occult malignancy is a prominent possibility which should be investigated. Our patient is unique in displaying both.
Basic clinical data such as the patient’s family history may be neglected in favour of sophisticated tests but remain crucial in evaluating the patient’s unique risk factors and directing diagnostic workup.
Unexplained digital ischaemia may be a rare presenting cutaneous sign of an occult cancer, in particular adenocarcinoma, and very rarely adenocarcinoma of the colon.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin 2009;59:73–98. 10.3322/caac.20005 [DOI] [PubMed] [Google Scholar]
- 2.Poszepczynska-Guigne E, Viguier M, Chosidow O et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol 2002;47:47–52. [DOI] [PubMed] [Google Scholar]
- 3.Hirschmann JV, Raugi GJ. Blue (or purple) toe syndrome. J Am Acad Dermatol 2009;60:1–20. 10.1016/j.jaad.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 4.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol 2008;143:180–90. 10.1111/j.1365-2141.2008.07323.x [DOI] [PubMed] [Google Scholar]
- 5.Suter LG, Murabito JM, Felson DT et al. The incidence and natural history of Raynaud's phenomenon in the community. Arthritis Rheum 2005;52:1259–63. 10.1002/art.20988 [DOI] [PubMed] [Google Scholar]
- 6.Spencer-Green G. Outcomes in primary Raynaud's phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998;158:595–600. [DOI] [PubMed] [Google Scholar]
- 7.Block JA, Sequeira W. Raynaud's phenomenon. Lancet 2001;357:2042–8. 10.1016/S0140-6736(00)05118-7 [DOI] [PubMed] [Google Scholar]
- 8.Madabhavi I, Revannasiddaiah S, Rastogi M et al. Paraneoplastic Raynaud's phenomenon manifesting before the diagnosis of lung cancer. BMJ Case Rep 2012;2012:pii: bcr0320125985. doi:10.1136/bcr.03.2012.5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schattner A. As sharp as Occam? Lancet 2009;373:1996 10.1016/S0140-6736(09)60696-6 [DOI] [PubMed] [Google Scholar]
- 10.Foo W, Young JM, Solomon MJ et al. Family history? The forgotten question in high-risk colorectal cancer patients. Colorectal Dis 2009;11:450–5. 10.1111/j.1463-1318.2009.01898.x [DOI] [PubMed] [Google Scholar]
- 11.Carrier M, Le Gal G, Wells PS et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med 2008;149:323–33. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen HT, Svaerke C, Farkas DK et al. Superficial and deep venous thrombosis, pulmonary embolism and subsequent risk of cancer. Eur J Cancer 2012;48:586–93. 10.1016/j.ejca.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 13.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12: 518–23. 10.1634/theoncologist.12-5-518 [DOI] [PubMed] [Google Scholar]
- 14.Le Besnerais M, Miranda S, Cailleux N et al. Digital ischemia associated with cancer. Results from a cohort study. Medicine (Baltimore) 2014;93:e47 10.1097/MD.0000000000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen BL, Petri C. Peripheral symmetrical gangrene in association with metastasizing carcinoma of the colon. Nord Med 1963;21:237–9. [PubMed] [Google Scholar]
- 16.Hawley PR, Johnston AW, Rankin JT. Association between digital ischaemia and malignant disease. BMJ 1967;3:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


