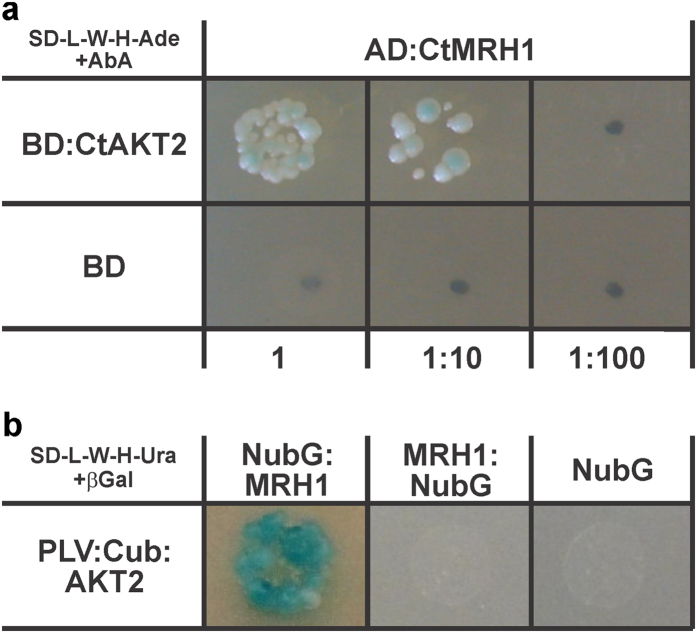
Figure 1. AKT2-MRH1 interaction in yeast.
(a) Yeast 2-Hybrid interaction test between the cytosolic C-terminal fragment of AKT2 fused to the DNA-binding domain as bait (BD:CtAKT2) and the cytosolic C-terminal fragment of the MRH1 kinase, derived from the cDNA library screening, fused to the activation domain as prey (AD:CtMRH1). Yeast growth on a highly selective medium lacking adenine, histidine, tryptophan, and leucine, supplemented with an antibiotic (AbA), indicate expression of several reporter genes. No growth and thus no reporter gene expression is observed if as bait only the DNA-binding domain (BD) is co-expressed with the AD:CtMRH1 prey. Images were taken after 5 days of growth. 5 μl of mated cultures were plated after dilution and incubated at 30 °C. (b) Split Ubiquitin interaction assay between the full-length AKT2 channel fused to the synthetic PLV transcription factor and the C-terminal domain of ubiquitin (PLV:Cub:AKT2 in a bait vector pMetYC-gate) and the full-length MRH1 kinase fused to the modified N-terminal domain of ubiquitin (NubG:MRH1 in prey vector pNX32-GW and MRH1:NubG in prey vector pXN32-GW). Reporter-gene expression can be observed only in one configuration. No auto-activity of the bait was observed. Liquid cultures of the L40 strain harboring both vectors were grown until the mid-logarithmic phase and standardized to OD600 = 1.0. Following a 1:10 dilution, 5 μl were plated on a medium containing 5-Brom-4-chlor-3-indoxyl-β-D-galactopyranosid but lacking leucine, tryptophan, adenine and uracil. Images were taken after 7 days of incubation at 30 °C. Results shown in (a) were repeated once and in (b) are representative for 2 independent repeats.