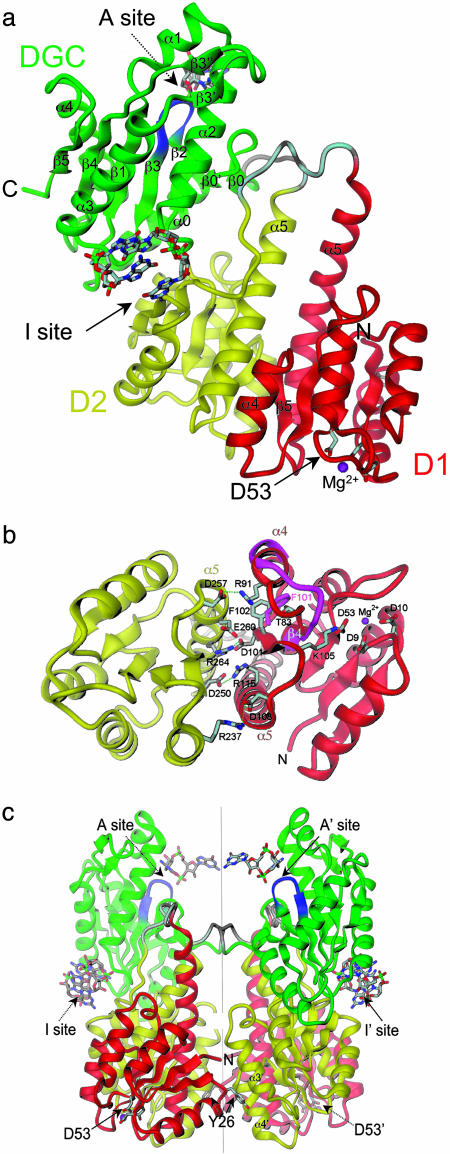
Fig. 1.
Crystal structure of PleD. (a) The monomer consists of three domains connected by disordered linker peptides (light gray). Domains D1 (residues 2–140, red) and D2 (residues 141–285, yellow) show the CheY-like fold. D1 carries the phosphoacceptor D53. The catalytic DGC domain (286–454) is shown in green. The GGEEF signature motif is located on the β-hairpin (blue) and constitutes part of the active site (A-site) to which a c-diGMP molecule is bound. Two c-diGMP molecules are found at the D2/DGC interface (I-site). (b) The D1(red)/D2(yellow) interface as viewed along the quasi-two-fold axis. Compared with the view in a, the structure has been rotated by 90° approximately around the horizontal. Ionic residues in the interface and residues implicated in activation (phosphoacceptor D53, K105, T83, and F102) are shown. The trace of the β4-α4 loop and F101 of phosphorylated FixJ [magenta; PDB ID code 1d5w (23)] is shown superimposed on D1. (c) The two monomers of the asymmetric unit form a two-fold dimer. The view is related by a –60° rotation about the dimer (vertical) axis with respect to the view in a. The c-diGMP molecules that are bound to sites A and A′ crosslink to another dimer above (see Fig. 2a).