Figure 3. Myosin phosphatase dephosphorylates eNOS at the inhibitory phosphorylation site, (pThr497): the role of PP1c and MYPT1 in the dephosphorylation of eNOSpThr497.
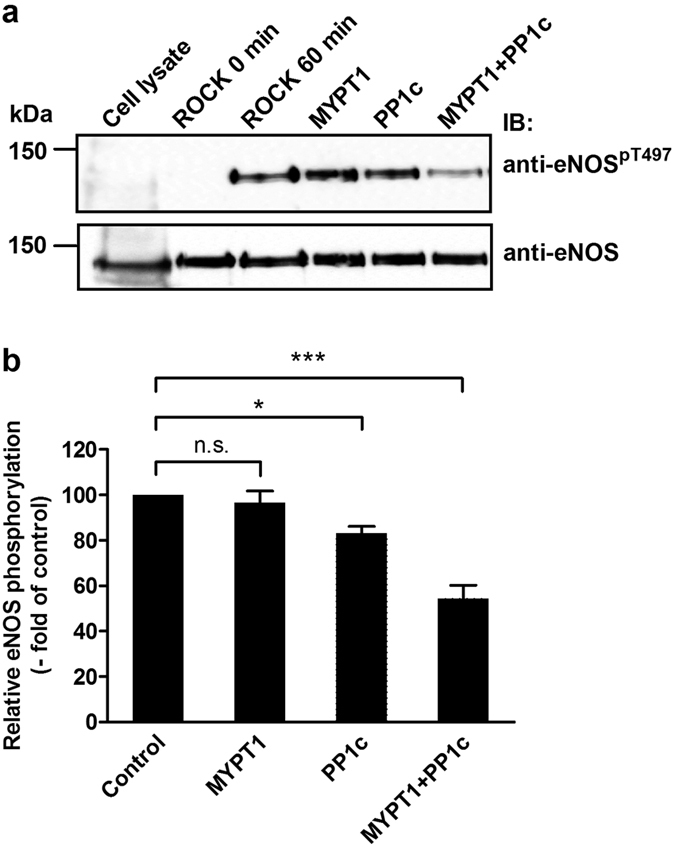
(a) c-myc-eNOS was isolated from the lysate of c-myc-eNOS expressing tsA201 cells on anti-c-myc-agarose beads and was phosphorylated by Rho-kinase (ROCK) for 60 minutes as described in Materials and Methods. Then, the resin was separated into four equal parts. Phosphorylated c-myc-eNOS was subjected to dephosphorylation for 15 min by FLAG-MYPT1 alone, PP1c alone, or by a mixture of PP1c and FLAG-MYPT1. The relative level of eNOSpT497 on anti- eNOSpT497 developed blots after phosphorylation by ROCK but before addition of the phosphatase components was considered as control (100%). Cropped images of representative Western blots are shown in (a). Uncropped, full-length blots are presented in Supplementary Information in Fig. S8. Densitometric analysis (b) of blots from three independent experiments are shown (means ± SEM (n = 3), *p < 0.05, **p < 0.01, One-way ANOVA, Newman-Keuls post-hoc testing).
