Figure 8. Regulation of eNOS activity and endothelial barrier function by phosphorylation-dephosphorylation.
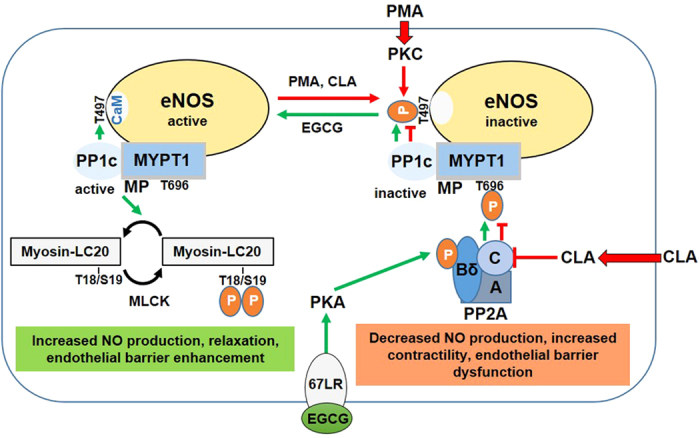
We have shown that myosin phosphatase (MP) interacts with eNOS via its myosin phosphatase target subunit-1 (MYPT1) which directs protein phosphatase-1 catalytic subunit (PP1c) for dephosphorylation of phospho-Thr497 allowing binding of Ca2+-calmodulin (CaM) and activation of eNOS. PMA and/or calyculin-A (CLA) increases the phosphorylation level of eNOS-Thr497 by activation of protein kinase C (PKC) and/or inhibition of PP2A, respectively (red lines). Suppression of PP2A activity results in increased phosphorylation of MYPT1 at Thr696 and inhibition of MP. Both PKC activation and MP inhibition contributes to the inactivation of eNOS via increased phosphorylation at Thr497 and thus interfering with CaM binding. Inactivation of eNOS accompanies by decreased NO production while the enhanced LC20 phosphorylation leads to increased contractility and thereby endothelial barrier dysfunction. Our present data suggest that the reactivation of eNOS and dephosphorylation of LC20 may be accomplished by an EGCG induced activation of both PP2A and MP involving the 67 kDa laminin receptor (67LR) and protein kinase A (PKA) in this phosphatase activation. The 67LR mediated action of EGCG implies activation of PKA which phosphorylates the Bδ subunit of PP2A resulting in PP2A activation and subsequent dephosphorylation of MYPT1 (at phospho-Thr696) and activation of MP (green lines). Then, the activated MP dephosphorylates both phosho-Thr497 in eNOS and phospho-Thr18/Ser19 in LC20 resulting in increased NO production and decreased contractility (relaxation) with endothelial barrier enhancement.
