Abstract
Guidelines for the management of acute pancreatitis (AP) are based on the Western experience, which may be difficult to extrapolate in India due to socioeconomic constraints. Hence, modifications based on the available resources and referral patterns should be introduced so as to ensure appropriate care. We reviewed the current literature on the management of AP available in English on Medline and proposed guidelines locally applicable. Patients of AP presenting with systemic inflammatory response syndrome are at risk of moderate-severe pancreatitis and hence, should be referred to a tertiary center early. The vast majority of patients with AP have mild disease and can be managed at smaller centers. Early aggressive fluid resuscitation with controlled fluid expansion, early enteral nutrition, and culture-directed antibiotics improve outcomes in AP. Infected pancreatic necrosis should be managed in a tertiary care hospital within a multidisciplinary setup. The “step up” approach involving antibiotics, percutaneous drainage, and minimally invasive necrosectomy instituted sequentially based on clinical response has improved the outcomes in this subgroup of patients.
Keywords: Acute pancreatitis, concepts, management
Introduction
The treatment of acute pancreatitis (AP) is essentially supportive as there is no available modality to reverse the progression of inflammation once initiated.[1] It is increasingly being recognized that maintenance of gut integrity is the most important determinant of outcome in AP.[2] Gut hypoxia secondary to hypovolemia and the systemic inflammatory response syndrome (SIRS) secondary to AP lead to increased translocation of gut flora. This further perpetuates the SIRS and predisposes to gut-related systemic and pancreatic infection. Hence, a large part of the management strategy in AP aims to reverse gut insult and promote gut mucosal integrity.
This review presents the current concepts in the management of AP. We also outline our institutional management strategy and have made recommendations relevant to the practice in India.
Diagnosis and Severity Scoring
The diagnosis of AP is made if two out of the three following criteria are present: Characteristic epigastric pain with radiation to the back, a 3-fold rise in serum amylase/lipase levels above baseline, and characteristic findings in abdominal imaging (ultrasonography/contrast-enhanced computed tomography [CT]/magnetic resonance imaging [MRI]).[3] Several severity scoring systems have been proposed to prognosticate AP and decide on an appropriate level of care at admission. The Ranson, Glasgow, APACHE II, and bedside index for severity assessment in pancreatitis are clinical- and biochemical- based scoring systems that are widely used and have sensitivities and specificities ranging from 70% to 90% in predicting severe AP (SAP).[4,5,6,7] However, these systems require 48 h for completion and include various parameters that may not be available at all centers.
The presence of organ failure and SIRS at presentation is increasingly being recognized as severity predictors in AP. In addition, the persistence of SIRS and organ failure despite 48 h of intense medical management is considered a poor prognostic sign.[8,9] SIRS at admission was found to have a sensitivity of 80.6% and a specificity of 65.9% for prediction of SAP.[4] The revised Atlanta Classification of 2012 recognizes organ failure at presentation and its persistence beyond 48 h after admission as criteria to measure severity [Table 1]. The modified Marshall's scoring system is used to define organ failure in the three most commonly affected organs in AP including the renal, cardiovascular, and respiratory systems [Table 2]. In the Indian context, peripheral hospitals are unlikely to have facilities to prognosticate AP severity based on the available criteria. We propose that patients presenting with SIRS be referred to a higher care center.
Table 1.
Revised Atlanta Classification[16]
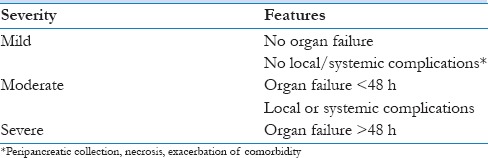
Table 2.
Modified Marshall scoring system for organ failure[16]

Fluid Resuscitation
Patients with AP are usually severely fluid depleted secondary to third spacing and vomiting. The hypovolemia and attending circulatory shock cause preferential diversion of blood from the splanchnic circulation to increase cardiac output and ensure adequate perfusion of vital organs.[2,10] Despite the ability of the intestine to increase oxygen extraction from the blood, prolonged hypoperfusion causes intestinal ischemia. This in turn leads to increased gut permeability and translocation of gut flora into the circulation perpetuating the already existing SIRS and predisposing to infection.[10] Following fluid resuscitation, adequacy of splanchnic perfusion is the last to be restored, and hence intestinal ischemia may well persist despite the patient appearing well hydrated.[2] In addition, the inflammatory state of AP increases the metabolic demand and hence, the requirement for oxygen necessitating a higher than normal perfusion. Hence, adequate fluid resuscitation is imperative early in the course of AP. The issue of fluid resuscitation needs to be discussed in the context of the amount of fluid and the rate at which it is to be administered, the type of fluid used, and the end points of resuscitation.
It is increasingly being recognized that controlled fluid expansion with early aggressive resuscitation results in a better outcome although without any impact on the incidence or extent of pancreatic necrosis. Two trials from the Mayo clinic confirmed that aggressive fluid resuscitation defined as administration of greater than one-third the 72 h fluid volume within the first 24 h resulted in a decreased incidence of organ failure, decreased SIRS scores, and lower Intensive Care Unit and in hospital stay.[11,12] These studies are, however, constrained by the retrospective nature over a 24 years period. On the other hand, there are studies that have demonstrated the pitfalls of early aggressive resuscitation and have hence recommended a controlled fluid expansion.[13,14,15,16,17] A retrospective, Swedish study demonstrated that administration of >4 L of fluid within the initial 24 h increased the incidence of lung complications including atelectasis, pneumonia, and effusions although not that of pulmonary edema.[13] It was however noted that patients who received a larger amount of fluids were sicker at presentation. A prospective, cohort, Spanish study involving 247 AP patients demonstrated that patients administered >4.1 L during the first 24 h had an increased incidence of pancreatic collections, persistent organ failure including respiratory and renal insufficiency.[18] We administer a bolus of 1 L normal saline (NS) in endoscopic retrograde (ER) followed by 150–200 ml/h for the first 48 h with allowances for patients with renal/cardiac failure.
The type of fluid to be used for initial resuscitation in AP is still a matter of debate. Crystalloids, colloids, albumin, and hypertonic saline have all been tried in either experimental or clinical studies. A randomized, controlled trial (RCT) comparing Ringer lactate (RL) and NS showed that the former was associated with lesser SIRS and greater reduction in C-reactive protein in patients with AP.[19] An experimental study on mice using Ringer's ethyl pyruvate solution (lactate replaced by ethyl pyruvate) showed that pyruvate being a potent antioxidant reduced pancreatic edema and necrosis consequently decreasing end-organ injury and improving survival in mice with necrotizing pancreatitis.[20] The use of hypertonic saline is evoking interest as, besides restoring the intravascular volume it enhances myocardial contractility and peripheral tissue perfusion, restores pancreatic microcirculation by shrinking edematous endothelial cells and inhibits leukocyte adhesion and transmigration. The beneficial effect of hypertonic saline in dampening the florid inflammatory response that accompanies AP has been demonstrated in a number of experimental studies.[20,21,22] However, the risk of central pontine myelinolysis and renal failure noted in burns patients precludes its widespread acceptance as a resuscitation solution in AP. We alternate RL and NS as the resuscitation fluid.
Endpoints of resuscitation should reflect the adequacy of splanchnic perfusion. However, as stated earlier, the splanchnic perfusion is the last to be restored and hence, the usual parameters used to gauge hydration may not be adequate. At present, there are no clinically acceptable markers for splanchnic perfusion. Apart from hemodynamic parameters and urine output, normalization of hematocrit (HCT) and blood urea nitrogen (BUN) has been associated with improved outcome.[19,23] Hence, we have adopted normalization of HCT and BUN as the end point of our resuscitation and aimed to do this within 48 h of admission.
Role of Imaging in Acute Pancreatitis
A contrast-enhanced CT scan remains the gold standard for imaging the pancreas. However, it is indicated at presentation only in case of a diagnostic dilemma. CT is most useful in differentiating a case of necrotizing pancreatitis from mild edematous pancreatitis.[24] However, a CT taken before 72 h may underestimate the degree of pancreatic necrosis as the extent may not be well defined until 72 h.[25,26] Optimal time for CT assessment is 72–96 h after the onset of symptoms.[27] Repeat studies are recommended where any invasive intervention is contemplated in patients who do not respond clinically to treatment or in case of changing clinical status.[27] CT guidance may be used for radiological drainage of infected collections or fine-needle aspiration of the necrotic pancreas to rule out infection.
An early ultrasound scan is indicated in all cases of pancreatitis to rule out biliary etiology particularly so in patients with a history and biochemical indicators of cholelithiasis. The presence of confirmed choledocholithiasis is an indication for ER cholangiopancreatography (ERCP) and stone extraction. The timing of ERCP depends on the clinical status of the patient. A meta-analysis of seven RCTs concluded that early ERCP (<72 h of pain onset) in cases of acute biliary pancreatitis without cholangitis did not result in a significant reduction in the overall complications (P = 0.38) or a decrease in mortality (P = 0.88).[28] Urgent therapeutic ERCP (<24 h of pain onset) is however advised in cases of biliary pancreatitis with cholangitis even in the absence of documented evidence of stone impaction.[27] However, in cases of biliary pancreatitis without cholangitis, MRCP and EUS may be used to confirm the presence of choledocholithiasis prior to ERCP.[29]
MRI is an alternate modality with an equivalent diagnostic value as CT when a contrast cannot be administered due to impending renal failure. T2-weighted image sequences identify the presence and extent of necrosis.[30] MRI has the additional advantage of avoiding ionizing radiation and is superior to CT in differentiating inflammatory fluid collections from liquefied necrosis.[31] However, MRI has the disadvantages of being difficult to use in acutely ill patient on multiple supports, longer acquisition times, and inability to perform simultaneous percutaneous procedures in contrast to CT.
Our patients get an ultrasound of the abdomen as the first investigation within 24 h of admission. A CT scan after 72 h of admission is performed in patients who clinically have severe pancreatitis. Follow-up CT scans are performed for patients failing to recover or those who deteriorate, with a view to diagnose and treat local complications. MRI is restricted to patients who have suspected biliary pancreatitis but equivocal ultrasound findings. Patients with biliary pancreatitis and confirmed stone in the bile duct have an ERCP within 48 h of admission, while those with cholangitis have one within 24 h.
Nutrition in Acute Pancreatitis
The traditional management of AP propounded the concept of “pancreatic rest” wherein patients were kept fasting as oral intake was thought to stimulate the pancreas and worsen pancreatitis. Hence, the patient had to be maintained on total parenteral nutrition (TPN) until oral intake was instituted. This concept was challenged after the pathophysiological mechanisms underlying the relationship between AP, gut permeability, and infection were elucidated.[2] Enteral nutrition (EN) was found to maintain mucosal integrity, prevent mucosal atrophy, and secures the tight junctions between mucosal cells thereby reducing mucosal permeability.[2,32] A recent meta-analysis of 8 RCTs showed that EN is superior to TPN in being associated with a lower incidence of death (P = 0.001), infectious complications (P = 0.004), organ failure (P = 0.02), and the need for surgical intervention (P = 0.003).[33] In addition, TPN risks line-related infections and other procedural complications and is expensive.[34] The issues that need to be addressed are the timing of initiation of nutrition, route of EN (nasogastric [NG] vs. nasojejunal [NJ]), and the composition of the diet.
The timing and route of EN are influenced by the presence of pain, vomiting, and the quantity of NG aspirates. A recent meta-analysis showed that EN started within 48 h of admission significantly reduced mortality (odds ratio [OR] =0.38) and incidence of pancreatic infections (OR = 0.49).[35] In addition, early EN (<48 h) was found to have a protective effect against the development of intra-abdominal hypertension in SAP.[36] A recent meta-analysis revealed that NG was equivalent to NJ feeding in terms of mortality (relative risk [RR] = 0.69), risk of aspiration (RR = 0.46), exacerbation of pain (RR = 0.94), and achieving nutritional targets (P = 0.97).[34,37] At our center, we attempt to initiate NG feeds within 48 h of admission. In the event of gastroparesis or increasing abdominal pain, an NJ feeding is initiated.
Various enteral formulations have been used in AP, but as yet there is no accepted standard formulation for initiation of feeds. Semi-elemental and polymeric feeds are the most commonly used formulations. Theoretically, semi-elemental feeds are considered most appropriate as they require lesser assimilation and stimulate the pancreas to a lesser extent but are far more expensive than polymeric feeds. Semi-elemental formulas contain small peptides, glucose polymers, and medium-chain fatty acids, while polymeric formulas contain nonhydrolyzed proteins, complex carbohydrates, and long-chain fatty acids. A meta-analysis including 1070 patients comparing semi-elemental and polymeric formulations found no statistical difference in feed intolerance between the two (P = 0.611).[38] However, a recent study showed a higher incidence of Chylous ascites (P < 0.05) in patients started on polymeric feeds.[39] The significance of this finding needs further confirmation. We commence NG feeding with a semi-elemental preparation.
The role of probiotics (nonpathogenic microorganisms) and immunonutrition in AP is controversial. The addition of probiotics was supposed to help stabilize the intestinal barrier and hence reduce infectious complications. A recent meta-analysis concluded that probiotics did not reduce infectious complications and hence is not recommended as standard of care.[40,41,42] Glutamine and omega 3 FFAs act as growth agents for dividing enterocytes and anti-inflammatory agents and are believed to decrease the impact of SIRS.[43] Two meta-analyses confirmed that glutamine and omega 3 FFA supplementation decreased the incidence of infectious complications, mortality, and resulted in a better outcome of patients with AP on TPN.[44,45] Hence, immunonutrition is recommended as a supplement to TPN but not EN.
Role of Antibiotics
Pancreatitis is essentially a sterile inflammation; hence, there is no justification for antibiotic prophylaxis in the acute setting. However, patients with necrotizing pancreatitis represent a unique subgroup wherein a third of patients develop infected pancreatic necrosis (IPN) with an associated morality >50%.[46] Much of the debate is centered on prophylaxis in this subgroup as it is presumed that antibiotics would prevent IPN and its attending complications. Twelve RCTs and an equivalent number of meta-analyses have addressed this issue but with no resulting consensus.[47] It is estimated that a large-scale RCT involving in excess of 3000 patients will be required to address the issue.[47] Logistical considerations are likely to preclude such a study. In addition, growing concerns about an increase in the prevalence of fungal infections and the development of multidrug-resistant organisms in patients with necrotizing pancreatitis caution against the indiscriminate use of antibiotics/antifungal in these patients.[48,49]
Mortality in acute necrotizing pancreatitis is linked to extent of necrosis, presence of infection and multiorgan failure.[46,50,51] The development of organ failure has been associated with impaired immune function and intestinal barrier dysfunction.[52,53] This suggests that patients with organ failure are more likely to develop infectious complications secondary to gut translocation. While <30% pancreatic necrosis are at low risk (10%) to develop infections, >50% necrosis is associated with an increased risk of organ failure (65%), infection, and need for interventions.[54,55,56] Hence, extensive necrosis may be an indication for prophylactic antibiotics. Selective digestive decontamination (SDD) is thought to benefit patients with necrotizing pancreatitis by decreasing potentially harmful gut flora. At present, there is no conclusive evidence from existing trials to recommend the routine use of SDD in AP.[57,58] Further controlled trials are indicated.
Current guidelines do not recommend the use of prophylactic antibiotics or antifungals in AP irrespective of the presence of necrosis.[3] However, we recommend that patients with persistent organ failure or those with necrosis >30% should be considered for prophylactic antibiotics. Therapeutic antibiotics should be administered based on sensitivities, and the duration should depend on clinical response and negative cultures. Empirical antibiotics are indicated in the presence of clinical sepsis. Simultaneous cultures (blood, urine, other body fluids, and tracheal), appropriate change of intravenous lines, and radiological investigations to identify the source of sepsis must be instituted.
Infected Pancreatic Necrosis
Mortality from IPN can be as high as 50%.[1] The traditional management of IPN involved open laparotomy, necrosectomy, and multiple drainages. This often required repeat procedures and was associated with a high morbidity and mortality. The current approach recommended is the “step up” approach which involves institution of culture-specific antibiotics, radiological intervention, and minimally invasive/endoscopic procedures sequentially depending on patient response.[59] The PANTER trial compared the “step up” approach to conventional open necrosectomy in IPN clearly demonstrated that the former was associated with a lower risk of major complications.[59] Interestingly, it also showed that about 40% of these patients were managed with percutaneous drainage alone and did not require surgical intervention.[59] If surgical intervention is indicated in IPN, the preferred technique is minimally invasive and based on the available local expertise.
Minimally invasive surgical techniques for necrosectomy include laparoscopic necrosectomy, video-assisted retroperitoneal debridement (VARD), and minimal access retroperitoneal pancreatic necrosectomy (MARPN).[60] MARPN requires prior insertion of a percutaneous drain under radiological guidance. The drain tract is progressively dilated to allow insertion of an endoscope. Necrosectomy is performed under direct vision. Unlike laparoscopic procedures, the coelom is not breached and hence, peritoneal contamination is avoided. However, it is unlikely to be successful in patients with multiple collections. VARD involves an open approach to the necrotic pancreas and subsequent insertion of an endoscope to perform the necrosectomy. Complications from the procedures described include bleeding, persistent infection, pancreatic fistula, damage to spleen and bowel, and incomplete debridement with the need for repeat procedures.
Endoscopic transgastric pancreatic necrosectomy is another alternative that is being increasingly used in select patients with IPN. It involves endoscopic access to the necrotic area through the posterior wall of the stomach. The TENSION trial comparing endoscopic to minimally invasive surgical necrosectomy is currently underway.[61] The results of this RCT will determine the role of endoscopic necrosectomy in IPN.
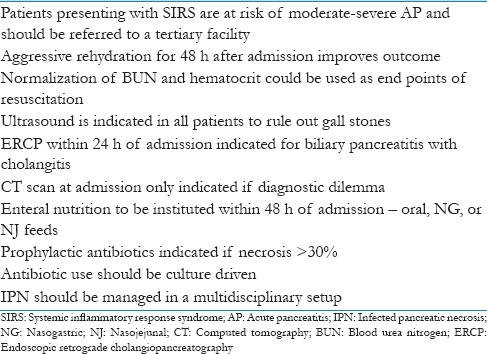
The “step up” approach with minimal access/endoscopic necrosectomy is the current standard of care in IPN. Patients with severe pancreatitis should be managed in a multidisciplinary setup with the availability of surgeons, gastroenterologists, radiologists, and intensivists. An early identification of patients with severe pancreatitis and those likely to develop complications and transfer to an appropriate facility is imperative.
Conclusion
We believe that the guidelines for AP should be framed based on the prevailing health resources in a country. Eighty percent of AP patients follow an indolent course and require minimal care which is available at every peripheral hospital. The challenge is to identify the remaining 20% early so that they can be referred to a higher facility. We recommend that patients with SIRS be transferred to a center with interest with AP. Unlike the other prognostic criteria, determination of SIRS is easy and can be done at all centers.
The management of AP involves optimal fluid resuscitation, early EN, appropriate antibiotics, and minimally invasive interventional techniques [Table 3]. The philosophy of management of IPN should be conservative with antibiotics, radiological intervention, and minimally invasive techniques instituted sequentially based on clinical response.
Table 3.
Key points
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.da Costa DW, Boerma D, van Santvoort HC, Horvath KD, Werner J, Carter CR, et al. Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg. 2014;101:e65–79. doi: 10.1002/bjs.9346. [DOI] [PubMed] [Google Scholar]
- 2.Flint RS, Windsor JA. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. HPB (Oxford) 2003;5:69–85. doi: 10.1080/13651820310001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15, 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 4.Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg 2013. 2013:367581. doi: 10.1155/2013/367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Johannes RS, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol. 2009;104:966–71. doi: 10.1038/ajg.2009.28. [DOI] [PubMed] [Google Scholar]
- 6.Simoes M, Alves P, Esparto H, Canha C, Miera E, Ferreira E, et al. Predicting acute pancreatitis severity: Comparison of prognostic scores. Gastroenterol Res. 2011;4:216–22. doi: 10.4021/gr364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–41. doi: 10.1038/ajg.2009.622. [DOI] [PubMed] [Google Scholar]
- 8.Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:1247–51. doi: 10.1016/j.cgh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–4. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh YY, Jeon WK, Cho YK, Kim HJ, Chung WG, Chon CU, et al. The effect of intestinal permeability and endotoxemia on the prognosis of acute pancreatitis. Gut Liver. 2012;6:505–11. doi: 10.5009/gnl.2012.6.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705–9. doi: 10.1016/j.cgh.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, et al. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9:770–6. doi: 10.1159/000210022. [DOI] [PubMed] [Google Scholar]
- 13.Eckerwall G, Olin H, Andersson B, Andersson R. Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: What have we learned and how can we do better? Clin Nutr. 2006;25:497–504. doi: 10.1016/j.clnu.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Mao EQ, Tang YQ, Li L, Qin S, Wu J, Liu W, et al. Strategy of controlling fluid resuscitation for severe acute pancreatitis in acute phase. Zhonghua Wai Ke Za Zhi. 2007;45:1331–4. [PubMed] [Google Scholar]
- 15.Mao EQ, Tang YQ, Fei J, Qin S, Wu J, Li L, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl) 2009;122:169–73. [PubMed] [Google Scholar]
- 16.Mao EQ, Fei J, Peng YB, Huang J, Tang YQ, Zhang SD. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl) 2010;123:1639–44. [PubMed] [Google Scholar]
- 17.Kuwabara K, Matsuda S, Fushimi K, Ishikawa KB, Horiguchi H, Fujimori K. Early crystalloid fluid volume management in acute pancreatitis: Association with mortality and organ failure. Pancreatology. 2011;11:351–61. doi: 10.1159/000328965. [DOI] [PubMed] [Google Scholar]
- 18.Nasr JY, Papachristou GI. Early fluid resuscitation in acute pancreatitis: A lot more than just fluids. Clin Gastroenterol Hepatol. 2011;9:633–4. doi: 10.1016/j.cgh.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–7.e1. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, et al. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med. 2004;32:1453–9. doi: 10.1097/01.ccm.0000130835.65462.06. [DOI] [PubMed] [Google Scholar]
- 21.Shields CJ, Winter DC, Sookhai S, Ryan L, Kirwan WO, Redmond HP. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Br J Surg. 2000;87:1336–40. doi: 10.1046/j.1365-2168.2000.01626.x. [DOI] [PubMed] [Google Scholar]
- 22.Horton JW, Dunn CW, Burnweit CA, Walker PB. Hypertonic saline-dextran resuscitation of acute canine bile-induced pancreatitis. Am J Surg. 1989;158:48–56. doi: 10.1016/0002-9610(89)90315-2. [DOI] [PubMed] [Google Scholar]
- 23.Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology. 2002;2:104–7. doi: 10.1159/000055899. [DOI] [PubMed] [Google Scholar]
- 24.Harris HW, Barcia A, Schell MT, Thoeni RF, Schecter WP. Necrotizing pancreatitis: A surgical approach independent of documented infection. HPB (Oxford) 2004;6:161–8. doi: 10.1080/13651820410033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balthazar EJ. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603–13. doi: 10.1148/radiol.2233010680. [DOI] [PubMed] [Google Scholar]
- 26.Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090–4. doi: 10.3748/wjg.v13.i22.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 28.Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, van Erpecum KJ, Gooszen HG. Early endoscopic retrograde cholangiopancreatography versus conservative management in acute biliary pancreatitis without cholangitis: A meta-analysis of randomized trials. Ann Surg. 2008;247:250–7. doi: 10.1097/SLA.0b013e31815edddd. [DOI] [PubMed] [Google Scholar]
- 29.Kapetanos DJ. ERCP in acute biliary pancreatitis. World J Gastrointest Endosc. 2010;2:25–8. doi: 10.4253/wjge.v2.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manikkavasakar S, AlObaidy M, Busireddy KK, Ramalho M, Nilmini V, Alagiyawanna M, et al. Magnetic resonance imaging of pancreatitis: An update. World J Gastroenterol. 2014;20:14760–77. doi: 10.3748/wjg.v20.i40.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo BC, Chinogureyi A, Shaw AS. Imaging acute pancreatitis. Br J Radiol. 2010;83:104–12. doi: 10.1259/bjr/13359269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotani J, Usami M, Nomura H, Iso A, Kasahara H, Kuroda Y, et al. Enteral nutrition prevents bacterial translocation but does not improve survival during acute pancreatitis. Arch Surg. 1999;134:287–92. doi: 10.1001/archsurg.134.3.287. [DOI] [PubMed] [Google Scholar]
- 33.Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, et al. Meta-analysis: Total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern Med. 2012;51:523–30. doi: 10.2169/internalmedicine.51.6685. [DOI] [PubMed] [Google Scholar]
- 34.Nally DM, Kelly EG, Clarke M, Ridgway P. Nasogastric nutrition is efficacious in severe acute pancreatitis: A systematic review and meta-analysis. Br J Nutr. 2014;112:1769–78. doi: 10.1017/S0007114514002566. [DOI] [PubMed] [Google Scholar]
- 35.Li JY, Yu T, Chen GC, Yuan YH, Zhong W, Zhao LN, et al. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: A meta-analysis. PLoS One. 2013;8:e64926. doi: 10.1371/journal.pone.0064926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun JK, Li WQ, Ke L, Tong ZH, Ni HB, Li G, et al. Early enteral nutrition prevents intra-abdominal hypertension and reduces the severity of severe acute pancreatitis compared with delayed enteral nutrition: A prospective pilot study. World J Surg. 2013;37:2053–60. doi: 10.1007/s00268-013-2087-5. [DOI] [PubMed] [Google Scholar]
- 37.Chang YS, Fu HQ, Xiao YM, Liu JC. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: A meta-analysis. Crit Care. 2013;17:R118. doi: 10.1186/cc12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrov MS, Loveday BP, Pylypchuk RD, McIlroy K, Phillips AR, Windsor JA. Systematic review and meta-analysis of enteral nutrition formulations in acute pancreatitis. Br J Surg. 2009;96:1243–52. doi: 10.1002/bjs.6862. [DOI] [PubMed] [Google Scholar]
- 39.Zhang SY, Liang ZY, Yu WQ, Wang ZE, Chen ZB, Zhang Y. Early enteral nutrition with polymeric feeds was associated with chylous ascites in patients with severe acute pancreatitis. Pancreas. 2014;43:553–8. doi: 10.1097/MPA.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 40.Oláh A, Belágyi T, Issekutz A, Olgyai G. Combination of early nasojejunal feeding with modern synbiotic therapy in the treatment of severe acute pancreatitis (prospective, randomized, double-blind study. Magy Seb. 2005;58:173–8. [PubMed] [Google Scholar]
- 41.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 42.Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18:R57. doi: 10.1186/cc13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong X, Liang CP, Gong S. Intravenous glutamine for severe acute pancreatitis: A meta-analysis. World J Crit Care Med. 2013;2:4–8. doi: 10.5492/wjccm.v2.i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asrani V, Chang WK, Dong Z, Hardy G, Windsor JA, Petrov MS. Glutamine supplementation in acute pancreatitis: A meta-analysis of randomized controlled trials. Pancreatology. 2013;13:468–74. doi: 10.1016/j.pan.2013.07.282. [DOI] [PubMed] [Google Scholar]
- 45.Jafari T, Feizi A, Askari G, Fallah AA. Parenteral immunonutrition in patients with acute pancreatitis: A systematic review and meta-analysis. Clin Nutr. 2015;34:35–43. doi: 10.1016/j.clnu.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267–73. doi: 10.1002/bjs.6447. [DOI] [PubMed] [Google Scholar]
- 47.Jiang K, Huang W, Yang XN, Xia Q. Present and future of prophylactic antibiotics for severe acute pancreatitis. World J Gastroenterol. 2012;18:279–84. doi: 10.3748/wjg.v18.i3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue P, Deng LH, Zhang ZD, Yang XN, Wan MH. Effect of antibiotic prophylaxis in acute necrotizing pancreatitis: Results of a randomised controlled trial. J Gasteroenterol Hepatol. 2009;24(5):736–42. doi: 10.1111/j.1440-1746.2008.05758.x. [DOI] [PubMed] [Google Scholar]
- 49.Su MS, Lin MH, Zhao QH, Liu ZW, He L, Jia N. Clinical study of distribution and drug resistance of pathogens in patients with severe acute pancreatitis. Chin Med J (Engl) 2012;125:1772–6. [PubMed] [Google Scholar]
- 50.Pal KM, Kasi PM, Tayyeb M, Mosharraf SM, Fatmi Z. Correlates of morbidity and mortality in severe necrotizing pancreatitis 2012. 2012:215193. doi: 10.5402/2012/215193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garg PK, Madan K, Pande GK, Khanna S, Sathyanarayan G, Bohidar NP, et al. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2005;3:159–66. doi: 10.1016/s1542-3565(04)00665-2. [DOI] [PubMed] [Google Scholar]
- 52.Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg. 2009;250:712–9. doi: 10.1097/SLA.0b013e3181bce5bd. [DOI] [PubMed] [Google Scholar]
- 53.Mentula P, Kylänpää-Bäck ML, Kemppainen E, Takala A, Jansson SE, Kautiainen H, et al. Decreased HLA (human leucocyte antigen)-DR expression on peripheral blood monocytes predicts the development of organ failure in patients with acute pancreatitis. Clin Sci (Lond) 2003;105:409–17. doi: 10.1042/CS20030058. [DOI] [PubMed] [Google Scholar]
- 54.Wig JD, Bharathy KG, Kochhar R, Yadav TD, Kudari AK, Doley RP, et al. Correlates of organ failure in severe acute pancreatitis. JOP. 2009;10:271–5. [PubMed] [Google Scholar]
- 55.Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut. 2005;54(Suppl 3):iii1–9. doi: 10.1136/gut.2004.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babu YR, Gupta R, Chandrasekaran P, Kang M, Bhasin DK. Maximum extent of pancreatic necrosis and not site of necrosis guide the therapeutic modality in severe acute pancreatitis. Gastroenterology. 2011;140(Suppl 1):S–1055. [Google Scholar]
- 57.Luiten EJ, Hop WC, Lange JF, Bruining HA. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995;222:57–65. doi: 10.1097/00000658-199507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Matsumura N, et al. Treatment outcome of selective digestive decontamination and enteral nutrition in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2007;14:503–8. doi: 10.1007/s00534-007-1216-7. [DOI] [PubMed] [Google Scholar]
- 59.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 60.Easler JJ, Zureikat A, Papachristou GI. An update on minimally invasive therapies for pancreatic necrosis. Expert Rev Gastroenterol Hepatol. 2012;6:745–53. doi: 10.1586/egh.12.48. [DOI] [PubMed] [Google Scholar]
- 61.van Brunschot S, van Grinsven J, Voermans RP, Bakker OJ, Besselink MG, Boermeester MA, et al. Transluminal endoscopic step-up approach versus minimally invasive surgical step-up approach in patients with infected necrotising pancreatitis (TENSION trial): Design and rationale of a randomised controlled multicenter trial [ISRCTN09186711] BMC Gastroenterol. 2013;13:161. doi: 10.1186/1471-230X-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]


