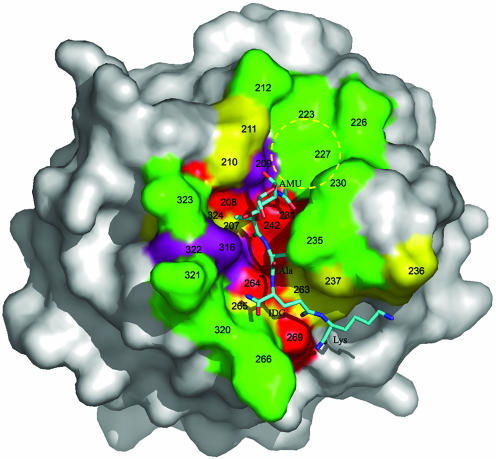
Fig. 4.
Surface analysis of the PGN-binding site of PGRP-Iα. The molecular surface is colored according to the percentage identities of residues lining the PGN-binding groove of PGRPs based on the sequence alignments in Fig. 6. Red, >80%; purple, 60–80%; yellow, 40–60%; and green, <40%. The bound MTP is shown in ball-and-stick representation, with carbon atoms in light blue, nitrogen atoms in dark blue, and oxygen atoms in red. A putative binding pocket for the GlcNAc moiety of natural PGNs, not present in the MTP fragment, is circled in yellow. Five of 16 MTP-contacting residues in the PGRP-IαC–MTP complex, at positions 208, 231, 242, 264, and 269, are highly conserved among PGRPs (>80% identity); only two contacting residues, at positions 235 and 266, are <40% conserved. AMU, MurNAc; IDG, d-isoglutamine.