ABSTRACT
Tumor-infiltrating lymphocytes (TILs) may represent a viable source of T cells for the biological treatment of patients with gliomas. Glioma tissue was obtained from 16 patients, tumor cell lines were established, and TILs were expanded in 16/16 cases using a combination of IL-2/IL-15/IL-21. Intracellular cytokine staining (ICS, IL-2, IL-17, TNFα and IFNγ production) as well as a cytotoxicity assay was used to detect TIL reactivity against autologous tumor cells or shared tumor-associated antigens (TAAs; i.e., NY-ESO-1, Survivin or EGFRvIII). TILs were analyzed by flow cytometry, including T-cell receptor (TCR) Vβ family composition, exhaustion/activation and T-cell differentiation markers (CD45RA/CCR7). IL-2/IL-15/IL-21 expanded TILs exhibited a mixture of CD4+, CD8+, as well as CD3+ CD4−CD8− T cells with a predominant central memory CD45RA−CCR7+ phenotype. TIL showed low frequencies of T cells testing positive for PD-1, TIM-3 and CTLA-4. LAG3 tested positive in up to 30% of CD8+ TIL, with low (1.25%) frequencies in CD4+ T cells. TIL cultures exhibited preferential usage of Vβ families and recognition of autologous tumor cells defined by cytokine production and cytotoxicity. IL-2/IL-15/IL-21 expanded TILs represent a viable source for the cellular therapy of patients with gliomas.
KEYWORDS: Brain tumor, cellular therapy, cytokines, glioma, TIL
Introduction
Cellular therapy using tumor-infiltrating lymphocytes (TILs) has been shown to mediate clinically relevant and durable response in patients with cancer. Regression of metastatic lesions has been documented in up to 70% of patients with melanoma who received adoptively transferred autologous TILs;1 40% of patients experienced complete regression of measurable lesions for at least 5 y following TIL treatment.2
The advent of a new screening approach for TIL-reactivity, i.e., mining whole-tumor DNA exome sequence data, allows to associate the clinical efficacy of TIL with their ability to recognize mutant tumor antigens.3 Not only anti-melanoma directed TILs but also mutation-specific T-cell responses to epithelial cancer have been shown to mediate clinically relevant anticancer directed CD4+ T helper-1 (TH-1) cell responses:4 for instance, a patient with cholangiocarcinoma achieved a decrease in tumor lesions after adoptive transfer of TILs, containing about 25% mutation-specific polyfunctional T(H)1 cells directed against a mutation in the erbb2 interacting protein (ERBB2IP). Upon subsequent disease progression, the patient was retreated with TIL containing a >95% population of mutation-reactive T(H)1 cells and experienced again tumor regression, providing evidence that a CD4+ T-cell response against a mutant antigen can be harnessed to mediate regression of metastatic epithelial cancer.5
Up to date, a limited number of studies were performed to offer biological therapy for patients with gliomas. Activated T cells have been able to control glioma growth using transgenic expression of IL-2, delivered by a retroviral vector.6 Alternatively, infusion of recombinant IL-2 into patients with brain tumors (n = 5) along with allogeneic cytotoxic T cells was performed. Two of five patients succumbed to the disease and three of five showed a clinical response defined by standard imaging technologies.7 In total, 12 clinical trials were conducted using either LAK cells, or targeted T-cell therapies.8-18 Quattrocci treated patients with gliomas with intra-lesional TIL and IL-219 leading to clinically relevant responses, i.e., one patient experienced a complete response, two patients a partial response and three patients progressed. Given the promising results from patients with melanoma and from patients with epithelial cancer, TIL therapy may also represent a viable option for the biological therapy of patients with glioma. However, the robust expansion of glioma-TIL has been challenging. The advent of reliable and successful expansion of TIL from patients with gliomas, using IL-2/IL-15/IL-21, may now facilitate the design of cellular treatment protocols for patients with CNS malignancies.
Results
Immunophenotype of TILs from glioma lesions
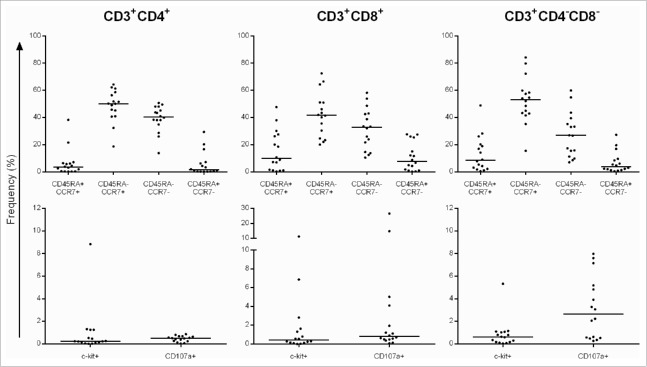
TILs and corresponding tumor cell lines from 16 patients with gliomas were successfully established (see patients' characteristics in Table S1). The composition of TIL was evaluated after a 4 week expansion using IL-2/IL-15/IL-21, allogeneic feeder cells and OKT3. TIL exhibited a median frequency of 94.5% CD3+ T cells; the median frequency of CD3+CD8+ and CD3+CD4+ T cells was 11.9% and 79.3%, respectively (Table 1). TIL exhibited a central (CCR7+ CD45RA−) and effector (CCR7− CD45RA−) memory T-cell phenotype in CD4+ T cells (median: 50.15% and 40.45%, respectively), in CD8+ T cells (median: 41.65% and 32.70%) and in the CD4−CD8− T-cell subset (double negative (DN), median: 53.10% and 26.75%). The median frequency of the precursor (CCR7+ CD45RA+) and terminally differentiated (CCR7− CD45RA+) T cells was found to be below 10% (Fig. 1, top panel). TILs exhibited a c-kit+ (CD117) median frequency of 0.24% in CD3+CD4+, 0.42% in CD3+CD8+ and 0.62% in DN T cells. The frequency of CD107a+ TIL (without antigenic stimulation) was 0.24% in CD3+CD4+, 0.80% in CD3+CD8+ and 2.65% in DN T cells (Fig. 1, bottom panel). We tested the identical TIL expansion protocol for the capacity to procure TIL from metastatic CNS metastatic lesions and obtained a similar T-cell phenotype (i.e., with the majority of T cells residing in the central 56%) and effector (27%) memory subsets (Fig. S1). TILs from metastatic lesions exhibited low c-kit (below 1%) and CD107a (3%) median frequencies. In order to test the impact of the Il-2/IL-15/IL-21-based expansion protocol on peripheral blood mononuclear cells (PBMCs), we expanded PBMCs from five patients with glioma in the presence of IL-2/IL-15/IL-21, stimulated with the tumor - associated antigen (TAA) NY-ESO-1, autologous feeder cells and OKT3 (Fig. S2). We did not observe an increase in the central memory subset (as observed in TILs), yet we detected the an increase in the effector memory T-cell subset with a median increase of 15–26% in the CD4+, CD8+ as well as in the DN (CD3+, CD4−, CD8+, DN) T-cell population. The TIL expansion protocol was also tested for expansion of PMBCs from eight healthy individuals. PBMCs were expanded with the cytokine cocktail IL-2/IL-15/IL-21 (and OKT3) without antigenic stimulation, or alternatively, with stimulation of a commonly recognized viral antigen, i.e., CMVpp65 (Fig. S3). Irrespective of the stimulation protocols (i.e., with or without CMVpp65 antigen stimulation), we observed a preferential expansion of effector memory T cells in CD4+, CD8+ as well as in DN T cells.
Table 1.
TIL phenotype.
| CD3+ | CD3+/ CD8+ | CD3+/ CD4+ | CD3+/ CD4−CD8− | CD3+/ CD4+CD8+ | |
|---|---|---|---|---|---|
| GBM-A | 75 | 11.4 | 85.2 | 2.92 | 0.47 |
| GBM-B | 73 | 90.1 | 8.31 | 1.07 | 0.5 |
| GBM-C | 94.6 | 12.4 | 85.4 | 2.13 | 0.25 |
| GBM-D | 73.5 | 24.2 | 71.2 | 3.71 | 0.99 |
| GBM-E | 98.2 | 4 | 89.9 | 2.63 | 3.53 |
| GBM-F | 99.6 | 3.58 | 89.9 | 6.15 | 0.38 |
| GBM-G | 94.3 | 0.52 | 77.8 | 21.6 | 0.08 |
| GBM-H | 92.7 | 38.5 | 52.6 | 5.8 | 3.1 |
| GBM-I | 98.5 | 44.2 | 2.81 | 52.9 | 0.08 |
| GBM-J | 98 | 1.16 | 91.7 | 6.34 | 0.84 |
| GBM-K | 85.2 | 33.3 | 49.4 | 10.8 | 6.5 |
| GBM-L | 94.2 | 81.3 | 12.2 | 6.02 | 0.46 |
| GBM-M | 99.9 | 0.02 | 92.5 | 7.39 | 0.1 |
| GBM-N | 99.7 | 95.4 | 0.24 | 4.33 | 0.07 |
| GBM-O | 82.5 | 0.59 | 84.7 | 14.6 | 0.12 |
| GBM-P | 99.4 | 1.37 | 80.7 | 17.8 | 0.1 |
| Median | 94.5 | 11.9 | 79.3 | 6.1 | 0.4 |
TILs were expanded from glioma lesions and tested after a 4 week expansion using IL-2, IL-15 and IL-21. The majority of TIL are CD3+, the numbers for CD8+, CD4+, CD4−CD8− and CD4+CD8+ (activated CD4+ T cells) represent the frequency of the respective T-cell population in the parental CD3+ T cells.
Figure 1.
Top: Frequency of TIL subpopulations based on CD45RA and CCR7 marker expression and bottom: c-kit, CD107a-positive T cells. Data represent the frequency of T-cell subpopulations in the parental CD4+, CD8+ and CD4−CD8− T cells. The majority of TIL resides in the CD45RA−CCR7+ (central memory) and CD45RA−CCR7− (effector memory) T-cell subsets. Each dot represent the TIL line from a single individual.
TILs were further characterized for expression of “activation-exhaustion” cell surface markers (Fig. 2)— exhibiting a low median frequency of four—1BB, CTLA-4, LAG-3 and TIM3—positive cells in CD3+CD4+, CD3+CD8+ as well as in the CD3+CD4−CD8− T-cell subpopulations, whereas PD-1+ T cells exhibited a median frequency of around 9% in CD3+CD4+ and 10% in CD3+CD8+ TIL. We did not observe Treg cells (Fig. S4) defined by CD4+CD25high CD127−Foxp3+ T cells or by CD4+CD25high CD39+CD73+ (around 1%) and CD4+CD25−CD39+CD73+ T cell (0.22%) marker analysis.
Figure 2.
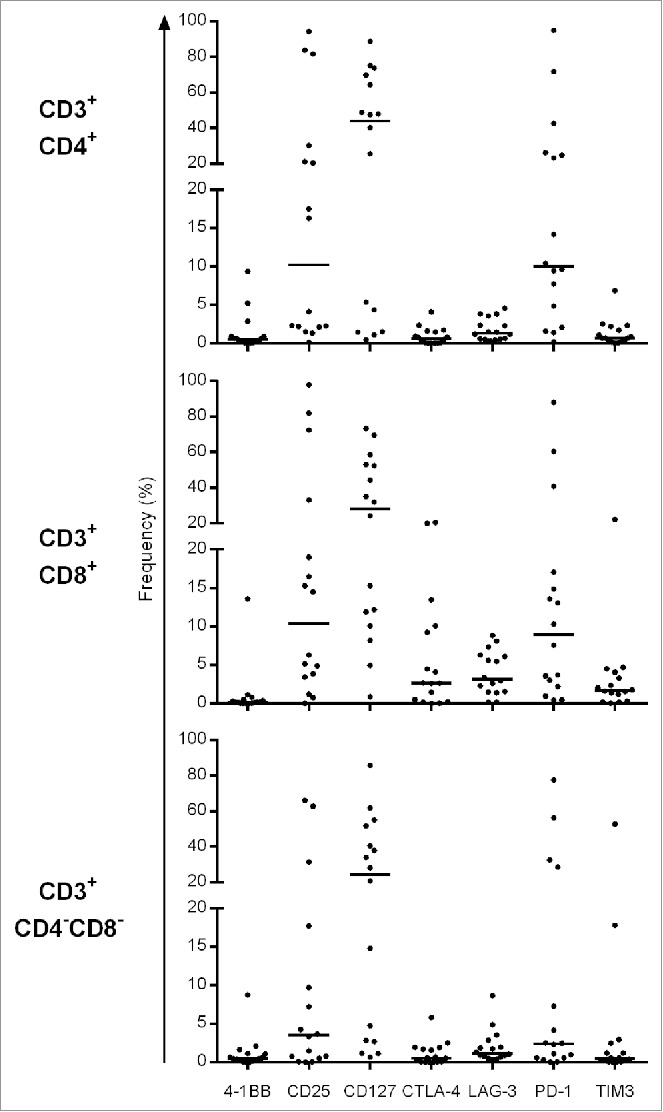
Frequency of exhaustion and activation marker (CD25, CD127, 4–1BB, PD-1, TIM-3, LAG-3 and CTLA-4)—positive populations in CD4+, CD8+ and CD4−CD8− TIL (16 individual patients).
Monoclonal and oligoclonal TIL TCR Vβ composition
T-cell receptor (TCR) Vβ families were analyzed by flow cytometry. Distinct TCR Vβ families in TIL from individual patients constituted up to 99% of TCRs (Table S2, highlighted in red), e.g., the TCRVβ2 which represented 99.6% of the CD3+CD4+ population in GBM-I TIL, the TCRVβ5.1 family representing 97% of GBM-I CD3+CD8+ TILs and the TCRVβ21,3 constituted 98.4% of the GBM-J CD3+CD4+ TILs. Some of the dominant TCR Vβ families were shown to be clonal, defined by TCR CDR3 length analysis and TCR sequencing (see Table S3).
TIL recognize autologous tumor cells
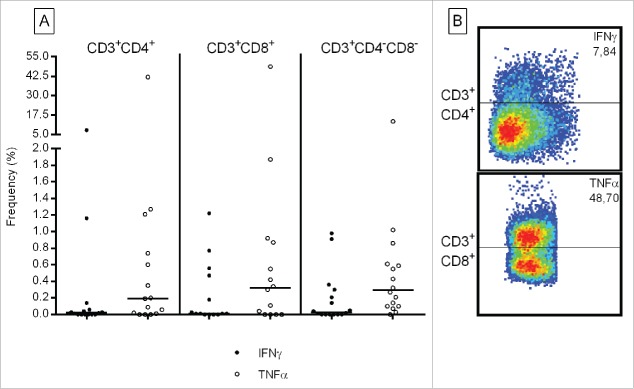
We tested whether TIL recognized autologous tumor cell lines defined by intracellular cytokine production. We observed IFNγ and TNFα production against autologous tumor cells in TIL from individual patients, e.g., up to 7.87% in CD3+CD4+ and up to 48.70% in CD3+CD8+ TIL (see Fig. 3 and for details Table S4). We were also able to link individual TCR Vβ families with tumor recognition, e.g., the TNFα production from GBM-D CD3+CD4+ TCRVβ2+ TILs (representing 33.3% TCRVβ2 T cells in CD3+CD4+ TIL) (Table S2 and Fig. S5): 3.5% T cells in the bulk CD3+CD4+ TIL exhibited TNFα production in response to autologous tumor cells. In contrast, the TCRVβ2 T-cell subpopulation showed a higher frequency (13.6%.) of TNFα producing CD3+CD4+ cells. Testing of TIL reactivity, using intracellular cytokine staining revealed selective IL-2 and IL-17 production of individual TIL lines against autologous tumor cells (Fig. S6 and Table S4). We also detected polyfunctional CD3+CD4+ T cells producing IFNγ + TNFα and IFNγ + IL-2 in response to autologous tumor cells (data not shown).
Figure 3.
Frequency of IFNγ and TNFα producing TIL in response to autologous tumor cells. Numbers represent the percentage of T cells specifically producing IFNγ (black circles) and TNFα (open circles) in the parental CD8+, CD4+ or (CD3+), CD4−CD8− TIL subsets. Each circle represent TIL from an individual patient. Medium values have been subtracted. Right panel: example flow cytometric analysis of CD4+ and CD8+ TIL producing cytokines directed against autologous tumor cells.
The potential of TIL to produce IFNγ was evaluated by incubating 105 TIL for 24 h with OKT3. 4/16 TIL lines did not produce IFNγ in response to TCR crosslinking (Table S5). Autologous tumor recognition was evaluated using a 3 d co-incubation of TIL with autologous tumor cells (data are shown after medium subtraction, Table S5). Tumor-specific cytokine production ranged from 13.32 to 1123.76pg/mL against autologous tumor cells in 11/16 TIL lines, which could partially be blocked (Table S5) using either the mAb W6/32 (anti-MHC-I Ab) or the mAb L243 (anti-HLA-DR). 9/16 TIL lines were tested in a standard Cr51 cytotoxicity assay (Fig. 4). Killing of autologous tumor cells could be detected in TILs at different effector:target (E:T) cell ratios ranging from 25:1 to 3.12:1, which could partially be blocked with an anti-MHC class I (w6/32) or—class II (L243) antibody (Table S5). Using the identical TIL expansion protocol, we have been able to expand TIL from a melanoma lesion recognizing the autologous tumor cell line (Fig. S7).
Figure 4.
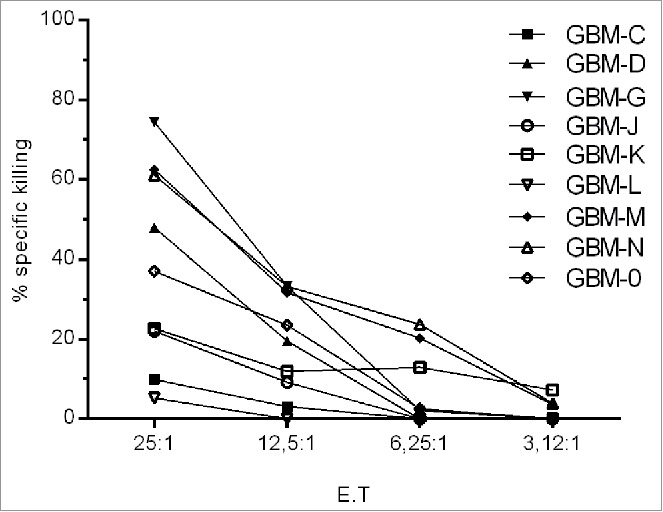
TIL recognize autologous tumor cells. A standard Cr51 cytotoxicity assay was performed using nine matched TIL/tumor samples, low reactivity to Daudi and K562 (data not shown).
MHC expression on tumor cells
Tumor cell lines were tested for expression of MHC molecules (Table S6) by incubation with/without IFNγ (−/+). 93.50% (mean) percent of tumor cells expressed MHC class I, and 8.17% (mean) exhibited HLA-DR+ tumor cells without IFNγ pre-incubation, whereas the frequency of MHC class I + tumor cells increased (median: 96.6%) and for HLA-DR up to 91.3% upon IFNγ stimulation. Not only the frequency of MCH-expressing tumor cells, yet also the number of MHC molecules/cells increased upon IFNγ incubation: 523 (median) MHC class I molecules per tumor cell without IFNγ were detected vs. 2,744 after IFNγ stimulation; similarly, the HLA-DR MFI (mean fluorescence intensity) showed an increase upon IFNγ incubation, i.e., 707 MFI/cells without IFNγ vs. 4,966 MFI with IFNγ.
TAAs recognition by TILs
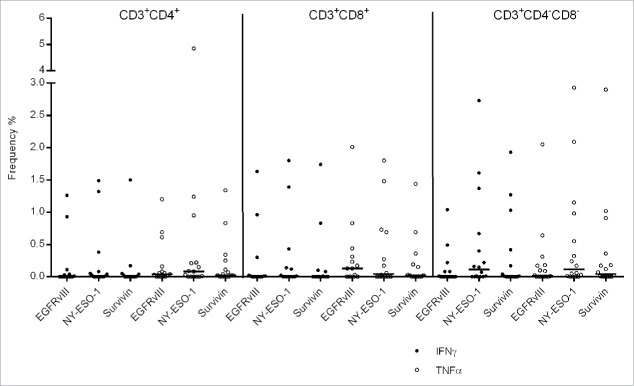
Besides the recognition of autologous tumor cells, we observed that TIL reacted against tumor associated antigens i.e., NY-ESO-1, survivin or EGFRvIII, characterized by cytokine production (data are shown after medium subtraction, the positive and negative control are shown in Fig. S8). Individual TIL lines were able to produce IFNγ up to 2.73% in DN T cells and TNFα up to 4.58% in the CD4+ T cells in response to NY-ESO-1 stimulation (Fig. 5 and Table S7). EGFRvIII stimulation resulted in 1.63% frequency of IFNγ producing and 2.05% frequency of TNFα producing CD8+ TIL. In order to evaluate whether the cytokine expansion protocol facilitates antigen-specific expansion in PBMCs from patients with cancer or from healthy individuals, PBMCs (from a patient with pancreatic cancer) were stimulated with two newly identified tumor-associated antigens, i.e., INO80E and UCHL3 (unpublished data, see Fig. S9) leading to 2% of CD8+ T cells reacting to a single epitope provided from INO80E and 0.40% of DN (CD3+, DN) T cells reacting against a single peptide from UCHL3. CMV was used as a surrogate antigens to expand PBMCs from two patients with glioma and from eight healthy individuals. Two different culture conditions were chosen in order to resemble the TIL expansion protocol, i.e., (i) PBMCs were restimulated twice with overlapping (15mer) peptides from CMVpp65 without OKT3 and (ii) alternatively with OKT3 (Figs. S10 and S11). The cytokine cocktail IL-2/IL-15/IL-21 resulted in CMV-pp65 specific INFγ and TNFα production in up to 35% and 58% of CD4+ T cells obtained from healthy individuals; and up to 47% and 52% in CD8+ T cells from patients with glioma.
Figure 5.
Frequency of IFNγ and TNFα producing CD4+, CD8+ and CD4−CD8− TIL in response to commonly shared tumor-associated antigens, i.e., EGFRvIII, survivin and NY-ESO-1. Medium responses have been subtracted.
Discussion
Glioma-derived TILs could be successful expanded in 16/16 cases, using IL-2, IL-15 and IL-21, this could not be achieved using a standard IL-2-based expansion protocol (data not shown). The benefit of combining IL-2, IL-15 and IL-21 in TIL expansion could be due to several factors, e.g., IL-21 has been shown to promote expansion of TIL with strong cytotoxic potential,2 it rescues CD8+ T cells from suboptimal antigenic stimulation and it has been shown to stimulate high affinity T cells without the need for CD8+ help;21 IL-15 and IL-21 may therefore aid to expand “better” T cells with increased frequencies of antigen-specific responses residing in long-term memory T-cell subsets.22-24 The preferential expansion of central memory T-cell subsets, defined by CD45RA-CCR7+ expression, appears to be associated with the nature of the T-cell source (e.g., TIL), since the IL-2/IL-15/IL-21 driven expansion of PBMCs from patients with glioma leads rather to expansion of effector memory T cells, defined by a CD45A-CCR7-profile. The IL-2/IL-15/IL-21 driven expansion of central memory T cells is not only related to glioblastoma; it can also be observed if solid cancer metastases are harvested from CNS (e.g., metastases from melanoma or colorectal cancer lesions, see Fig. S2); these TIL are reactive against autologous tumor cells (Fig. S7). However, we were also able to show that the combination of IL-2/IL-15/IL-21 leads to strong expansion of antigen-specific T cells, reflected in the expansion of CMV-reactive T cells in PBMCs from patients with glioma (Fig. S3).
Not only the profile of antitumor-directed T cells may be relevant, yet also their potency to resist immune-suppressive factors in situ, since the local milieu of brain tumors is characterized by (i) Th2-oriented immune responses25 (ii) production of immuno-suppressive cytokines, i.e., TGFβ, IL-1026 and (iii) inhibition of T-cell proliferation.27 In concert with IL-21, IL-15 has been shown to overcome immunosuppressive effects on tumor-reactive T cells, in part by neutralizing negative effects of tumor-associated macrophages28 and by increasing antitumor activity in CD8+ T cells.29
IL-2, IL-15 and IL-21-expanded TILs from glioma lesions could be used for cellular therapy with the hope to achieve clinically relevant responses as it has been reported for patients with melanoma or other solid cancers.30, 31 Non-myeoloablative regimens, i.e., fludarabine and cyclophosphamide, have been reported to facilitate the transfer of T cells,32 along with Interleukin-2 application, to ensure survival of transferred cells.33 Central memory T cells (CD45RA−CCR7+) have been shown to exhibit stronger proliferative potential in adoptive TIL therapy and are most likely the best candidates to provide long-term antitumor reactivity.34, 35 The passive transfer of T cell clones, directed against tumor-associated antigens, showed that tumor-reactive T cells persist, acquire a central-memory phenotype and are able to mediate long-term (up to 3 y) remissions in individual patients.36 Similar results were obtained using ex vivo expanded polyclonal T-cell products.37 Our data show that the majority of glioma TIL reside in the CD45RA−CCR+ central memory T-cell subset and may therefore represent a viable source for adoptive cellular therapy along with antitumor reactivity: individual TIL clones reported here (e.g., GBM-J: 98.4% CD4+Vβ21.3 or GBM-N: 90.70% CD8+Vβ7.1 T cells; Table S2) show specific reactivity directed against autologous tumor cells (Fig. 4) defined by cytokine production and/or by cytotoxicity.
Note that absent IFNγ production in individual TIL lines (see Table S5A) does not preclude the clinical transfer of these T cells. Particularly, TIL that showed low or absent IFNγ production (Table S5A) exhibited strong cytolytic reactivity in the Cr51 release assay (Fig. 4). More recent data showed that TIL are able to target cancer mutations and that these T cells mediate clinically relevant regressions.5 A similar mechanism may be clinically relevant for patients with glioma, supported by the notion that the frequency of mutations in glioma has been associated with stronger T-cell infiltrates.38
The method described in the current report allows now to reliably expand TIL, that react against autologous glioma cells and to perform tumor DNA exome-sequencing with the subsequent analysis of TIL-defined tumor-associated targets. A caveat of the current study is that recognition of autologous tumor cells was tested using short-term (autologous) established tumor cell lines, which may not represent the diversity of tumor cells presented in the resected cancer lesion.39 Tumor cells with “stem cell like properties” have been identified in cancer and “stem cell-associated targets,” including CD133, may represent clinically relevant TAA in some brain tumors40-42 leading to target-specific T-cell responses.43 A dense infiltration of CD8+ TIL into CNS tumors has been reported to be correlated with increased survival44 making TIL infusion an attractive therapy option for adoptive cellular therapy.45,46 A dose-escalating clinical phase I TIL trial at Karolinska for patients with gliomas is currently being prepared.
Materials and methods
For more detailed information concerning the Materials and methods section, please refer to the supplementary data.
Diagnosis and patients
Sixteen patients (Table S1) with gliomas were enrolled. The study was approved by the regional ethical review board at Karolinska Institutet, Stockholm, Sweden (Dnr: 2013/576–31).
TIL expansion
Glioma tumor tissue was harvested in the course of tumor surgery at the Department of Neuro-Oncology at Karolinska Hospital. Tumor tissue was immediately transferred to Cellgro (CellGenix, Catalog Number: 20801-0500) medium supplemented with 5% pooled human AB serum (Innovative Research, Catalog Number: IPLA-SERAB-14668). Tumor tissue was dissected into fragments (approximately 1–2 mm3) using a sterile scalpel (for TIL production) or processed into a tissue homogenate using a Medimachine (BD, California, USA Cat:340588) for tumor cell line production. Tissue fragments of the cell suspension were washed two times with ice-cold PBS and cultured in 24-well plates in GMP Serum-free DC medium (CellGenix, Catalog Number: 20801-0500) plus 5% pooled human AB serum (Innovative Research, Catalog Number: IPLA-SERAB-14668) supplemented with recombinant IL-2 (1000 IU/mL) (Prospec, Catalog Number: cyt-209-b)/IL-15 (10 ng/mL) (Prospec, Catalog Number: cyt-230-b)/IL-21 (10 ng/mL) (Prospec, Catalog Number: cyt-408-b). Medium was changed when necessary. Irradiated (55Gry) feeder cells (allogeneic PBMCs) at the ratio of 1 (feeder cells):10 (TILs) was added on day 7. TILs were transferred into six-well plates; as they covered >70% of the 24-well surface, they were further expanded in G-Rex flasks (Wilson Wolf, Catalog Number: 800400S) using 30 ng OKT3/mL and irradiated (55Gry) allogeneic feeder cells at the ratio of 1 (feeder cells):5 (TILs).
Tumor cell generation
Tumor tissue was dissected into fragments (approximately 1–2 mm3) as described above using a sterile scalpel or processed into a tissue homogenate using a Medimachine (BD, Catalog Number: 340588). The tissue fragments of the cell suspension were washed two times in PBS and then cultured in T25 flasks (Thermo Fisher Scientific, Inc., Catalog Number:734-0045) using RPMI 1640 l-glutamine (2 mM) with antibiotics (penicillin,100 IU/mL and streptomycin, 100 µg/mL) (Life Technologies, Catalog Number: 61870) plus 20% Fetal Bovine Serum (Life technologies, Catalog Number: 10270-106). TILs and tumor cell from metastatic brain tumor lesion could be expanded with the same protocol.
Immunophenotype, “activation/exhaustion” and Tregs analysis
TILs and PBMCs were stained using the same Ab cocktail as described previously47 and in detail in the supplementary data. Analysis was performed using a FACS Aria flow cytometer (BD Biosciences, Stockholm, Sweden) and FlowJo software.
Intracellular cytokine staining
At day 1, a 12 h stimulation of 1 million cells at 37 °C was performed using EGFRvIII, NY-ESO-1, survivin and CMV-pp65 peptides. TILs were also exposed to autologous tumor cells for 12 h and tested for cytokine production. Stimulation was stopped by incubating cells at 4 °C. At day 2, TILs were washed and stained using the same Ab cocktail and protocol as described47 (in detail in the supplementary data).
Vβ test and PCR-based TCR CDR3 analysis
TCR Vβ frequency staining was performed using the β Mark TCR Vβ Repertoire Kit (Beckman Coulter, Catalog Number: PN IM3497) along with co-staining with anti-CD3 PE-Cy7 (BD Biosciences, Catalog Number: 300316), anti-CD4+ Krome Orange (Beckman coulter, Catalog Number: PN A96417) and anti-CD8a APC-Cy7 (BD Biosciences, Catalog Number: 557834). After washing, a FACS Aria flow cytometer (BD Biosciences, Stockholm, Sweden) was used for acquisition and data analysis was performed by FlowJo software. TCR CDR3 analysis was performed using the TCR Vβ panel as described before.48 Through PCR, the TCR Vβ CDR3 analysis was performed using the TCR Vβ family specific primer set panel described48,49 (see for details, the supplementary data).
TIL-tumor cell co-cultures and IFNγ ELISA
TILs were exposed to autologous tumor cells in 96-well plates in triplicates and T-cell responses were blocked with W6/32 (Sigma-Aldrich, Catalog Number: 84112003) or L243 (Biolegend, Catalog Number: 307602) at 1 µg/well. Cells were seeded at 20,000 TILs/well at an E:T ratio of 10:1. Supernatants were harvested at days 3 and 7 and tested for IFNγ by ELISA (Mabtech, Catalog Number: 3420–1H-20). The capacity of TIL to produce IFNγ was tested by placing 105 TILs/well (in 0.2 mL IL-2, IL-15, IL-21— containing TIL-medium) in triplicates in 96 well plates containing 30 ng/mL OKT-3. Control wells contained TIL without OKT-3; supernatants were harvested after 24 h and tested for IFNγ production by ELISA (see above).
Cytotoxic assay
A chromium 51 (Cr51) release assay was performed to measure lytic activity. Serial dilutions of TIL were performed ranging from 25:1 to 3.12:1 TIL: tumor cell ratio. Tumor cells without TILs and TIL (plus medium only) served as the negative control (spontaneous release), 5% Triton X-100 (Sigma-Aldrich, Catalog Number: 93426) and Cr51-labeled cells served as the positive control (maximum release). The number of target cell per well was 10,000 cells. Target cells were labeled with 250 mCi/mg Na2CrO4 (PerkinElmer, Catalog Number: NEZ030005MC) at 37 °C for 1 h. After washed with PBS for three times, target cells were re-suspended in in RPMI 1640 l-glutamine (2 mM) with antibiotics (penicillin,100 IU/mL and streptomycin, 100 µg/mL) (Life Technologies, Catalog Number: 61870) plus 10% Fetal Bovine Serum (Life Technologies, Catalog Number: 10270-106) at a concentration of 105 cells/mL and then distributed to 96-wells plate at 10,000/well. TIL and tumor cells were incubated for 4 h at 37 °C and 5% CO2. After 4 h, 50 µL of supernatant was transferred from each well to an Isoplate 96-well sample plate (PerkinElmer, Catalog Number: 6005040) containing 200 µL OptiPhase Super Mix (PerkinElmer, Catalog Number: 1200-439); the sample plate was sealed by a plastic cover (PerkinElmer, Catalog Number: 1450-461) and mixed for a few seconds to ensure a homogenous solution. Cr51 radioactivity was measured using a 1450 Microβ Trilux scintillation counter with the Microβ workstation software 4.0 (PerkinElmer, MA, USA). For each effector:target (E:T) cell ratio, the percentage of specific lysis was calculated as follows:
Ab block killing
TILs were co-incubated with radioactively labeled autologous tumor cells in the E:T ratio of 12.5:1 in the presence of W6/32 (anti MHC-I Ab) or L243 (anti HLA-DR Ab) in a 4 h Cr51 release assay (1µg mAb/well), the non-MHC directed mAb directed against the first component of complement C1q (mAb 242G3) served as the isotype control antibody.
MHC-I and -II expression
Tumor cells were cultured in RMPI, supplemented with 10% FBS and recombinant IFNγ (ImmunoTools, Catalog Number: 352712) was added at 100 U/mL. After 4 d, tumor cells were collected and washed once with PBS, then tumor cells (with or without IFNγ stimulation) were stained with anti HLA-ABC-FITC (Beckman Coulter, Catalog Number: IM1838U) or the HLA-DR APC-Cy7 (BD Biosciences, Catalog Number: 335831) antibodies for 15 min and washed once with PBS once. A FACS Aria flow cytometer (BD Biosciences, Stockholm, Sweden) was used for data acquisition, analysis was performed using FlowJo software. In order to translate MFI into the number of MHC molecules per tumor cell, the quantum MFSF Kit (Bangs Laboratories Inc., Catalog Number: 555A) was used to enumerate molecules/cells. Results were reported as percent of tumor cells showing MHC class I or HLA-DR expression as well as the number of molecules/cell (for MHC class I), or HLA-DR (MFI).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by a grant from the Söderberg foundation to MM and also by Karolinska Institutet.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM et al.. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002; 298:850-4; PMID:12242449; http://dx.doi.org/ 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR et al.. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res: Off J Am Assoc Cancer Res 2011; 17:4550-7; PMID:21498393; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E et al.. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19:747-52; PMID:23644516; http://dx.doi.org/ 10.1038/nm.3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu YC, Yao X, Crystal JS, Li YF, El-Gamil M, Gross C, Davis L, Dudley ME, Yang JC, Samuels Y et al.. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res: Off J Am Assoc Cancer Res 2014; 20:3401-10; PMID:24987109; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS et al.. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344:641-5; PMID:24812403; http://dx.doi.org/ 10.1126/science.1251102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo F, Barzon L, Franchin E, Pacenti M, Pinna V, Danieli D, Zanusso M, Palu G. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther 2005; 12:835-48; PMID:15891772; http://dx.doi.org/ 10.1038/sj.cgt.7700851 [DOI] [PubMed] [Google Scholar]
- 7.Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol, Immunother: CII 1997; 45:77-87; PMID:9390198; http://dx.doi.org/ 10.1007/s002620050405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes RL, Arbit E, Odaimi M, Pannullo S, Scheff R, Kravchinskiy D, Zaroulis C. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol/Hematol 2001; 39:31-42; PMID:11418300; http://dx.doi.org/ 10.1016/S1040-8428(01)00122-6 [DOI] [PubMed] [Google Scholar]
- 9.Dillman RO, Duma CM, Ellis RA, Cornforth AN, Schiltz PM, Sharp SL, DePriest MC. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J Immunother 2009; 32:914-9; PMID:19816190; http://dx.doi.org/ 10.1097/CJI.0b013e3181b2910f [DOI] [PubMed] [Google Scholar]
- 10.Jacobs SK, Wilson DJ, Kornblith PL, Grimm EA. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: phase I trial. Cancer Res 1986; 46:2101-4; PMID:3512079 [PubMed] [Google Scholar]
- 11.Dillman RO, Duma CM, Schiltz PM, DePriest C, Ellis RA, Okamoto K, Beutel LD, De Leon C, Chico S. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother 2004; 27:398-404; PMID:15314549; http://dx.doi.org/ 10.1097/00002371-200409000-00009 [DOI] [PubMed] [Google Scholar]
- 12.Sankhla SK, Nadkarni JS, Bhagwati SN. Adoptive immunotherapy using lymphokine-activated killer (LAK) cells and interleukin-2 for recurrent malignant primary brain tumors. J Neuro-oncol 1996; 27:133-40; PMID:8699235; http://dx.doi.org/ 10.1007/BF00177476 [DOI] [PubMed] [Google Scholar]
- 13.Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, Pierz DM, Chen DK, Budzilovich GN, Ransohoff J. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer 1995; 76:840-52; PMID:8625188; http://dx.doi.org/ 10.1002/1097-0142(19950901)76:5%3c840::AID-CNCR2820760519%3e3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 14.Boiardi A, Silvani A, Ruffini PA, Rivoltini L, Parmiani G, Broggi G, Salmaggi A. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol, Immunother: CII 1994; 39:193-7; PMID:7923250; http://dx.doi.org/ 10.1007/BF01533386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blancher A, Roubinet F, Grancher AS, Tremoulet M, Bonate A, Delisle MB, Calot JP, Pourreau C, Franks C, Ducos J et al.. Local immunotherapy of recurrent glioblastoma multiforme by intracerebral perfusion of interleukin-2 and LAK cells. Eur Cytokine Network 1993; 4:331-41; PMID:8117934 [PubMed] [Google Scholar]
- 16.Jeffes EW 3rd, Beamer YB, Jacques S, Silberman RS, Vayuvegula B, Gupta S, Coss JS, Yamamoto RS, Granger GA. Therapy of recurrent high grade gliomas with surgery, and autologous mitogen activated IL-2 stimulated killer (MAK) lymphocytes: I. Enhancement of MAK lytic activity and cytokine production by PHA and clinical use of PHA. J Neuro-Oncol 1993; 15:141-55; PMID:8509819; http://dx.doi.org/2643685 10.1007/BF01053935 [DOI] [PubMed] [Google Scholar]
- 17.Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg 1989; 70:175-82; PMID:2643685; http://dx.doi.org/ 10.3171/jns.1989.70.2.0175 [DOI] [PubMed] [Google Scholar]
- 18.Merchant RE, Grant AJ, Merchant LH, Young HF. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer 1988; 62:665-71; PMID:2840186; http://dx.doi.org/ 10.1002/1097-0142(19880815)62:4%3c665::AID-CNCR2820620403%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 19.Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, Gudeman S, Varia MA. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neuro-Oncol 1999; 45:141-57; PMID:10778730; http://dx.doi.org/ 10.1023/A:1006293606710 [DOI] [PubMed] [Google Scholar]
- 20.Santegoets SJ, Turksma AW, Suhoski MM, Stam AG, Albelda SM, Hooijberg E, Scheper RJ, van den Eertwegh AJ, Gerritsen WR, Powell DJ Jr et al.. IL-21 promotes the expansion of CD27+ CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells. J Trans Med 2013; 11:37; PMID:23402380; http://dx.doi.org/ 10.1186/1479-5876-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imataki O, Ansen S, Tanaka M, Butler MO, Berezovskaya A, Milstein MI, Kuzushima K, Nadler LM, Hirano N. IL-21 can supplement suboptimal Lck-independent MAPK activation in a STAT-3-dependent manner in human CD8(+) T cells. J Immunol 2012; 188:1609-19; PMID:22238455; http://dx.doi.org/17363542 10.4049/jimmunol.1003446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler MO, Lee JS, Ansen S, Neuberg D, Hodi FS, Murray AP, Drury L, Berezovskaya A, Mulligan RC, Nadler LM et al.. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res: Off J Am Assoc Cancer Res 2007; 13:1857-67; PMID:17363542; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1905 [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol 2005; 175:2261-9; PMID:16081794; http://dx.doi.org/15477598 10.4049/jimmunol.175.4.2261 [DOI] [PubMed] [Google Scholar]
- 24.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA 2004; 101:15154-9; PMID:15477598; http://dx.doi.org/ 10.1073/pnas.0406649101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol 1996; 105:344-52; PMID:8706344; http://dx.doi.org/ 10.1046/j.1365-2249.1996.d01-753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodmer S, Strommer K, Frei K, Siepl C, de Tribolet N, Heid I, Fontana A. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol 1989; 143:3222-9; PMID:2809198; http://dx.doi.org/0022-1767/89/14310-3222802.00/03257792 [PubMed] [Google Scholar]
- 27.Miescher S, Whiteside TL, de Tribolet N, von Fliedner V. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg 1988; 68:438-48; PMID:3257792; http://dx.doi.org/ 10.3171/jns.1988.68.3.0438 [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Song L, Wei J, Courtney AN, Gao X, Marinova E, Guo L, Heczey A, Asgharzadeh S, Kim E et al.. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Investigat 2012; 122:2221-33; PMID:22565311; http://dx.doi.org/ 10.1172/JCI59535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC et al.. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA 2004; 101:1969-74; PMID:14762166; http://dx.doi.org/ 10.1073/pnas.0307298101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009; 21:233-40; PMID:19304471; http://dx.doi.org/ 10.1016/j.coi.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. New Engl J Med 2008; 358:2698-703; PMID:18565862; http://dx.doi.org/ 10.1056/NEJMoa0800251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF et al.. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol: Off J Am Soc Clin Oncol 2008; 26:5233-9; PMID:18809613; http://dx.doi.org/ 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA 2002; 99:16168-73; PMID:12427970; http://dx.doi.org/ 10.1073/pnas.242600099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Investigat 2008; 118:294-305; PMID:18060041; http://dx.doi.org/ 10.1172/JCI32103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 2003; 4:225-34; PMID:12563257; http://dx.doi.org/ 10.1038/ni889 [DOI] [PubMed] [Google Scholar]
- 36.Chapuis AG, Thompson JA, Margolin KA, Rodmyre R, Lai IP, Dowdy K, Farrar EA, Bhatia S, Sabath DE, Cao J et al.. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci USA 2012; 109:4592-7; PMID:22393002; http://dx.doi.org/ 10.1073/pnas.1113748109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler MO, Friedlander P, Milstein MI, Mooney MM, Metzler G, Murray AP, Tanaka M, Berezovskaya A, Imataki O, Drury L et al.. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Trans Med 2011; 3:80ra34; PMID:21525398; http://dx.doi.org/23864165 10.1126/scitranslmed.3002207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LA, Appin C, Park Y, Scarpace L, Mikkelsen T, Cohen ML et al.. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res: Off J Am Assoc Cancer Res 2013; 19:4951-60; PMID:23864165; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Na Rev Immunol 2013; 13:101-17; PMID:23334244; http://dx.doi.org/ 10.1038/nri3369 [DOI] [PubMed] [Google Scholar]
- 40.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63:5821-8; PMID:14522905 [PubMed] [Google Scholar]
- 41.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004; 64:7011-21; PMID:15466194; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1364 [DOI] [PubMed] [Google Scholar]
- 42.Ernst A, Hofmann S, Ahmadi R, Becker N, Korshunov A, Engel F, Hartmann C, Felsberg J, Sabel M, Peterziel H et al.. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res: Off J Am Assoc Cancer Res 2009; 15:6541-50; PMID:19861460; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0695 [DOI] [PubMed] [Google Scholar]
- 43.Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM et al.. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res: Off J Am Assoc Cancer Res 2010; 16:800-13; PMID:20103663; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012; 72:1070-80; PMID:22266112; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3:666-75; PMID:12951585; http://dx.doi.org/ 10.1038/nrc1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P et al.. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol: off Am Soc Clin Oncol 2009; 27:5944-51; PMID:19858404; http://dx.doi.org/ 10.1200/JCO.2008.19.6147 [DOI] [PubMed] [Google Scholar]
- 47.Meng Q, Liu Z, Rangelova E, Poiret T, Ambati A, Rane L, Xie S, Verbeke C, Dodoo E, Del Chiaro M et al.. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J Immunother 2016; 39:81-9; PMID:26849077; http://dx.doi.org/ 10.1097/CJI.0000000000000111 [DOI] [PubMed] [Google Scholar]
- 48.Magalhaes I, Vudattu NK, Jager E, Maeurer MJ. Tumor antigen-specific T-cells are Present in the CD8alphaalpha+ T-cell effector-memory pool. J Immunother 2008; 31:840-8; PMID:18833003; http://dx.doi.org/ 10.1097/CJI.0b013e31818883a1 [DOI] [PubMed] [Google Scholar]
- 49.Ahmed RK, Poiret T, Ambati A, Rane L, Remberger M, Omazic B, Vudattu NK, Winiarski J, Ernberg I, Axelsson-Robertson R et al.. TCR+CD4-CD8- T cells in antigen-specific MHC class I-restricted T-cell responses after allogeneic hematopoietic stem cell transplantation. J Immunother 2014; 37:416-25; PMID:25198529; http://dx.doi.org/ 10.1097/CJI.0000000000000047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.