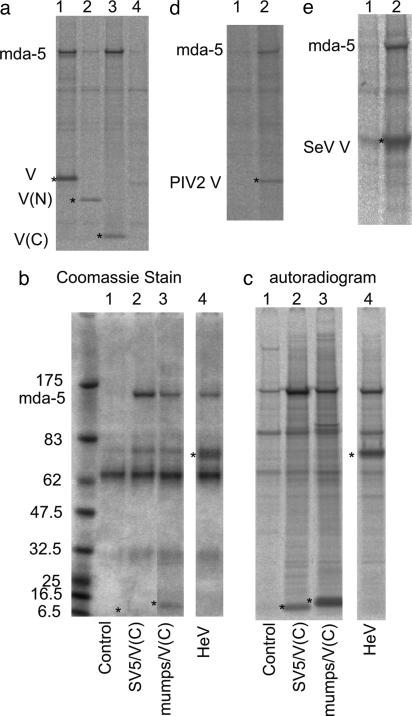
Fig. 2.
mda-5 interacts with the C terminus of the SV5 V protein and with other paramyxovirus V proteins. (a) Cells were transfected for 36 h with vectors expressing nontagged mda-5 (lane 4), together with myc-tagged versions of SV5 V(W3A) (lane 1), the N terminus of V (1–174) [V(N); lane 2], or the C terminus of V (126–222) [V(C); lane 3], or a control “empty vector” (lane 4) and immunoprecipitated with anti-myc antibody. (b and c) Coomassie stain and autoradiogram, respectively, of immunoprecipitates, by using anti-myc antibody, of cells transfected for 36 h with plasmids expressing nontagged mda-5, together with a control “empty vector” (lane 1) or plasmids expressing myctagged versions of the C terminus of SV5 [SV5/V(C); lane 2] and of mumps [mumps/V(C); lane 3] or myc-tagged HeV V (lane 4). (d and e) Autoradiograms of immunoprecipitates from cells transfected with a plasmid that express the nontagged mda-5, together with a control plasmid (lane 1), a plasmid expressing PIV 2/V(d, lane 2), or a plasmid expressing myc-tagged SeV V (e, lane 2). Cells were metabolically labeled with 35[S]methionine for 1 h before the myc-tagged proteins being immunoprecipitated with the 9E10 mAb and the V protein of hPIV-2 immunoprecipitated with the mAb Pk2 (32). *, the bands corresponding to the V proteins or derivatives. Proteins were separated on 4–12% polyacrylamide gradient gels (Invitrogen).