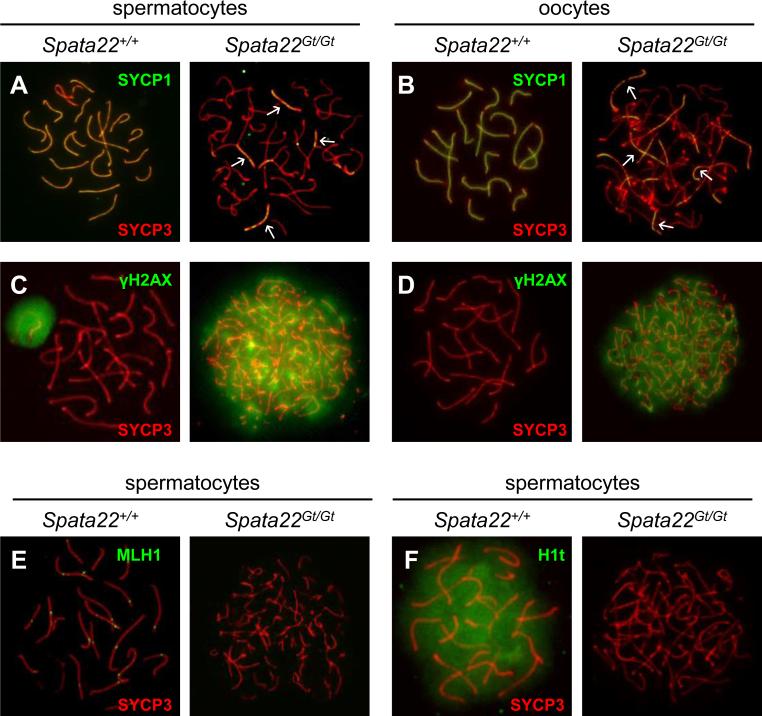
Figure 4. Synapsis and DNA repair are severely impaired in Spata22Gt/Gt spermatocytes and oocytes.
(A, B) Co-localization of SYCP1 (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (A) and oocytes (B). The pattern of yellow color representing co-labeling with anti-SYCP1 and anti-SYCP3 represents the extent of synapsis (white arrows). (C, D) Detection of γH2AX (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (C) and oocytes (D). The pattern of SYCP3 determines the prophase I substage. Whereas wild type germ cells reach pachynema, mutant cells appear to arrest at zygonema. The pattern of γH2AX reflects DSB formation and repair; persistence of γH2AX suggests DSBs are induced but not repaired in mutant germ cells. (E) Localization of MLH1 (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes. MLH1 is found at crossover sites in wild-type pachytene spermatocytes but is undetectable in mutant spermatocytes. (F) Detection of the testis-specific histone H1t (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes. H1t, which becomes expressed by mid-pachynema in wild type spermatocytes, is never observed in mutant cells, confirming that arrest takes place prior to the mid-pachytene stage. Original magnification: ×630.