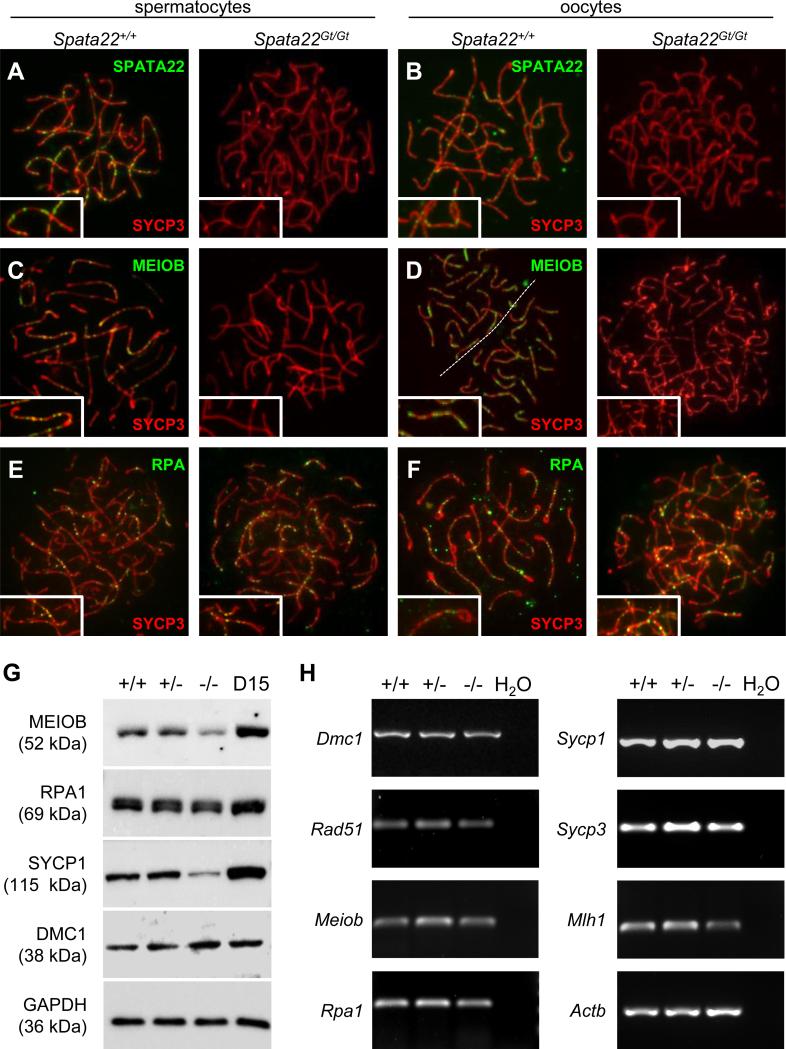
Figure 7. SPATA22 deficiency impairs localization and/or stability of proteins required for meiotic recombination and synapsis.
(A, B) Localization of SPATA22 (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (A) and oocytes (B).(C, D) Localization of MEIOB (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (C) and oocytes (D). (E, F) Localization of RPA (green) and SYCP3 (red) in wild type (left) and Spata22Gt/Gt (right) spermatocytes (E) and oocytes (F). Whereas SPATA22 and MEIOB are absent in mutant meiocytes, RPA localization remains unaffected. (G) Immunoblotting analysis of protein extracts prepared from D15 wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−) testes, as well as D15 B6 control testes, with antibodies against MEIOB, RPA1, SYCP1 and DMC1. Expression of GAPDH (bottom panel) served as a loading control to normalize protein expression across genotypes. The molecular weight of each protein (in kDa) is indicated to the left of the corresponding immunoblot. (H) Analysis of RNA extracts prepared from D15 wild type (+/+), heterozygous (+/−), and homozygous mutant (−/−) testes. Transcripts for all meiotic genes assessed were amplified by RT-PCR. The name of the gene evaluated is indicated to the left of each corresponding panel. Expression of Actb was used to normalize the expression of each gene across genotypes. H2O, water control. D, postnatal day.