ABSTRACT
Candida albicans remains the main etiological agent of candidiasis, as this otherwise normal commensal of humans is capable of causing active infection in immune- and medically-compromised patients. The high morbidity and mortality rates associated with candidiasis, coupled with the emergence of drug resistance demand the development of novel therapeutic strategies. However, there is a paucity of selective targets that can be exploited in the development of new antifungals. Contrary to conventional antibiotics that kill or curtail growth, specifically targeting virulence mechanisms represents an attractive option for antifungal drug development. In C. albicans, a growing body of research over the last few decades has provided important insights into its virulence factors and their contribution to the pathogenesis of candidiasis. Of these, filamentation is the one that has received the most attention and perhaps shows the most promise as a target for new anti-virulence strategies to combat C. albicans infections.
KEYWORDS: anti-virulence therapeutics, Candida albicans, candidiasis, filamentation
Infections caused by Candida albicans and current treatment options
Candida spp. are normal colonizers of the gastrointestinal and genitourinary tracts, as well as the skin of healthy humans and, as commensals, inflict little to no damage to the host.1,2 Normal anatomical barriers and innate defense mechanisms (i.e. saliva in the oral cavity, neutrophils in the circulation) are normally sufficient to help maintain the commensal status.3,4 However, when normal microbiota balance is disturbed or immunity is impaired, Candida overgrowth can lead to host damage and establishment of the opportunistic infection.1,2 Over the last few decades, dramatic improvements in a variety of medically related procedures and therapies have led to a dramatic increase in survival of critically ill patients. However, these advances have also increased the population of immunocompromised patients, patients with hematologic disorders/malignancies, surgery, transplantation, patients in ICU and those undergoing anti-cancer and cytotoxic therapy, diabetes, hemodialysis, mechanical ventilation, parenteral nutrition, aging patients and recipients of artificial joints and prosthetic devices, among others, as well as notably HIV/AIDS patients; whom are all vulnerable to invasive fungal infections.5,6
Among all pathogenic species of Candida, C. albicans remains the most common etiological agent of candidiasis and accounts for approximately 50% of bloodstream Candida isolates in the United States.6,7 As a pathogen, C. albicans can cause a variety of infections that range from superficial to life-threatening invasive candidiasis.6 Oropharyngeal candidiasis remains the most common oral manifestation in HIV patients, and is also frequent in head and neck cancer patients, as well as transiently in individuals treated with antibiotics or corticosteroids.8-10 Additionally, 3 out of 4 women are likely to experience at least one event of vulvovaginal candidiasis (VVC) over the course of their lifetime, and ∼5% of the female population suffers from recurrent infections (RVVC).11 Invasive candidiasis affects more than 250,000 people worldwide every year and it is estimated to cause over 50,000 deaths.12 Deep-seated infections may remain localized or lead to secondary candidemia (i.e., dissemination through the bloodstream). Candidemia may occur after disruption of mucosal integrity after surgery or by direct inoculation through medical devices such as catheters, with circulating yeasts able to colonize and infect virtually any internal organ of the host leading to invasive disease.6,13 Importantly, candidemia now ranks as the third most common cause of health care-associated bloodstream infection (BSI) and is the leading cause of BSIs in the intensive care unit (ICU),7 with incidence rates of 2–14 cases per 100,000 persons.12
Treatment of candidiasis, from superficial to invasive infections, relies on a limited drug arsenal, composed of 3 major classes of antifungal drugs: polyenes, azoles and echinocandins.14,15 However, this arsenal is compromised by problems of selectivity, toxicity, and the development of resistance. The polyenes were the first class of antifungal drugs introduced into the clinic (in the1950s) and include amphotericin B and nystatin. These amphipathic molecules bind to the ergosterol in fungal cell membranes and form pores, leading to leakage of intracellular constituents and impaired protein traffic through the membrane.14,16 Amphotericin B also exists as a large extramembranous aggregate that acts as a fungicidal sponge that kills yeast by simply binding and extracting ergosterol.17,18 Despite great fungicidal activity against Candida, amphotericin B use is limited by its associated hepatotoxicity and nephrotoxicity,14 especially in severely ill ICU patients and those undergoing hemodialysis, which actually corresponds to an important risk group for the development of invasive candidiasis. More recently, two lipid-based formulations of amphotericin B, lipid complex (ABLC) and liposomal amphotericin B, with reduced toxicity and improved pharmacokinetics, were introduced for clinical use; however they are significantly more expensive than conventional amphotericin B and not widely used.19 Triazoles, including both first generation (fluconazole and itraconazole) and second generation (voriconazole and posaconazole), comprise the most commonly used class of antifungals. The introduction of triazoles in clinics during the 1980s and 1990s revolutionized medical mycology 19 and until the introduction of the echinocandins fluconazole was the drug of choice in the treatment of most C. albicans infections. All triazoles are inhibitors of C14α-lanosterol demethylase, a key enzyme involved in the biosynthesis of ergosterol; thus disturbing the integrity of the fungal cell membranes.14 Because fluconazole is readily absorbed, has good biodistribution and the greatest penetration into the cerebrospinal fluid and vitreous humor, it is the treatment of choice for several invasive forms of candidiasis, including cystitis, ocular and central nervous system dissemination.7 Unfortunately, a major problem with fluconazole is the emergence of resistance (including cross-resistance against multiple azole derivatives), mostly through the development of point mutations in the target enzyme or by overexpression of specific efflux pumps.20 The newest class of antifungals available in the clinic is the echinocandins, released in early 2000. Echinocandins, including anidulafungin, micafungin and caspofungin, inhibit the synthesis of β-1,3 glucan, a major polysaccharide of the fungal cell wall and, therefore, is the only antifungal class to target an exclusive fungal component.14 Echinocandins are the most selective and least toxic antifungal drugs and, following regulatory approval, their use in both prophylaxis and treatment has grown substantially. As a consequence of increased drug exposure, resistance has emerged and been linked to indiscriminate echinocandin use.21 Acquired echinocandin resistance has now been reported in single isolates belonging to most Candida species, including C. albicans,21 with the main mechanism of resistance identified as mutations in the FKS1 gene encoding the target enzyme, glucan synthase.22,23
Targeting virulence represents an attractive new approach for antifungal drug development
As mentioned above, since fungi are eukaryotic, the development of antifungal agents is complicated by the limited number of selective targets that can be exploited for drug development, leading to the exceedingly short arsenal of drugs. Moreover, the antifungal pipeline is sparse and there are few new drugs in sight.19,24 Irrespective of their targets and mechanism of action, conventional antifungal drugs act by inhibiting growth or killing the fungal cells; in either case they pose a high degree of selective pressure which is ultimately responsible for the emergence of resistance. An attractive alternative is to target virulence factors that are specific to C. albicans. For the purpose of this review, we define virulence as the ability of the fungus to cause active disease, and virulence factor as a C. albicans component or process that actively participates in causing damage to host tissues or promotes infection.25,26 In essence, such an anti-virulence approach will “disarm” C. albicans from its capacity to cause infection, thereby preventing the transition to the pathogenic state or reverting it back to harmless commensal status. Of course, development of such anti-virulence approaches requires specific knowledge of C. albicans pathogenicity, which is complex and multifactorial in nature and, as an opportunistic pathogen, depends on a delicate balance between virulence attributes and host responses.3 Fortunately, this has been an area of intensive research during the last few decades, leading to the identification of multiple factors and mechanisms that represent major contributors to the pathogenic potential of this fungus, as recently reviewed.27 Thus, the time is now right to try to take advantage of all the information accumulated during these years and to apply this accrued knowledge to more translational endeavors for the development of novel antifungal therapeutics.
The concept of anti-virulence therapy emerged in the bacterial field mainly as a potential solution to overcome antibiotic drug resistance, and has gained traction in the last few years, having been fully embraced as a desirable alternative that is increasingly being explored and exploited in the development of novel classes of antibacterials.28,29 However, the same feeling is not true in the case of antifungal drug development, which is still dominated by a somewhat unrealistic expectation that any new antifungal must have a broad spectrum of action and be able to kill or inhibit the growth of most, if not all, pathogenic fungi. In stark contrast, and by definition, anti-virulence strategies are effective against a much more limited range of microorganisms (only those that share the same virulence factor) and thereby display a narrow spectrum of action.29
There are many different advantages of targeting virulence as opposed to cell viability and some of these may be particularly appealing in combating fungal infections. Perhaps most importantly, in the case of antifungals, due to the paucity of selective targets in the eukaryotic fungal cell, the identification of virulence factors can substantially expand the number of potential targets that can be exploited for antifungal drug development and, in the process, lead to entirely new classes of drugs with new mechanisms of action. Moreover, because of its narrow spectrum of action, and anti-virulence approach should not alter the natural host's microbiota, and this could be of critical importance in the case of normal commensals such as C. albicans. Finally, stripping fungal cells of their virulence without threatening their existence should exert a much reduced selection pressure for drug resistance mutations, which has been a major problem in C. albicans, particularly with respect to the azole derivatives.
Targeting filamentation for antifungal drug development in C. albicans
C. albicans filamentation and its role in virulence
C. albicans is polymorphic and capable of undergoing reversible morphological transitions between single-cell ellipsoid budding yeast and different filamentous forms, including pseudohyphae (chains of elongated cells with constrictions at the septa) and true hyphae (which posses parallel walls and grow by continuous apical extension and septation).30-32 These morphogenetic conversions greatly contribute to disease, and although both yeast and filaments are normally observed during infection, they play distinct functions with yeast cells participating in early phases of the infectious process (i.e. adhesion and dissemination) and filamentous forms responsible for invasion and damage to tissues and organs, leading to pathology and potentially death.33-35 Thus, because of its predominant role during the disease process, of all putative C. albicans virulence factors, without any question filamentation is the one that has received the most attention and has been the focus of a majority of the research conducted on this fungus. Early experiments revealed that genetically defined mutant strains locked in the yeast morphology, and therefore unable to filament, were avirulent in a murine model of C. albicans invasive infection.36 Subsequent studies using regulatable strains in which morphogenetic conversions could be controlled both in vitro and in vivo provided compelling evidence for the role of filamentation in the progression to active infection,35,37 as well as genetic validation that filamentation could be targeted in the development of new antifungal agents.38 Furthermore, although filamentation is coordinately regulated with other virulence factors 39-41, the observation that virulence of the Δhgc1 mutant (see below) is heavily attenuated in the murine model of invasive candidiasis provides further credence to the notion that filamentation per se constitutes a major virulence factor.42 Although a systematic screen of a collection of C. albicans deletion strains pointed to a lack of correlation between filamentation and virulence,43 we note that the authors looked only at infectivity (not active disease), and we and others have shown that yeast cells can extravasate and infect target organs without necessarily lead to mortality.13,35 Figure 1 shows extensive filamentation of C. albicans cells associated with characteristic invasive kidney lesions in infected mice with hematogenously disseminated candidiasis.
Figure 1.
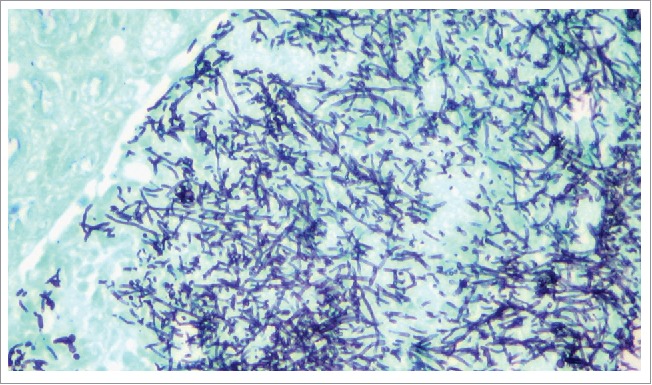
C. albicans filamentation in kidney tissues of infected mice. Fungal elements are visualized in kidney tissues from mice with invasive candidiasis by using the Grocott-Gomori methenamine-silver (GMS) stain.
Control of C. albicans filamentation by multiple signaling pathways
Filamentation can be triggered by a multitude of stimuli that act through multiple complex signal transduction pathways, and readers are referred to excellent, detailed and in depth reviews on this topic.44,45 Signals able to stimulate filamentation include neutral pH, 5% CO2, body temperature (37°C), serum, nutrient availability as well as embedded and microaerophilic conditions. Certain human hormones such as estrogen and progesterone are also known to stimulate filamentation and biofilm formation leading to vulvovaginal candidiasis (VVC).46 The ability of C. albicans to respond to a multitude of signals allows its adaptation to the environmental conditions presented by the host. The filamentation process in C. albicans is tightly regulated, while simultaneously being highly dynamic, occurring under a wide range of environmental conditions.2,45,47 The intricate regulation of hyphal initiation and extension is further complicated by the convergence of multiple signaling pathways on the same or different transcription factors, as well as several transcription factors converging on similar targets. Filamentation is subject to both negative and positive regulation. Negative regulation is carried out by Tup1 acting in combination with Nrg1 or Rfg1, or by Rbf1 alone.45 Indeed, modulation of NRG1 using a tetracycline regulatable promoter has revealed that C. albicans cells overexpressing NRG1 grow exclusively as yeast under any hypha-enducing condition.35 Furthermore, deletion strains of C. albicans lacking TUP1, NRG1 or both are locked into a filamentous form.48,49 Positive regulators of filamentation include Efg1, Cph1, Tec1, Czf1, Hgc1, Ume6, Brg1, and Rim101.45,50-53 These positive regulators are able to respond to signals transmitted though different filamentation pathways or are able to further relay the signal from multiple pathways. Efg1 is responsible for inducing filamentation in response to pathways stimulated by N-Acetyl Glucosamine (GlcNac), pH (by interacting with Rim101), CO2 (through the activation of the cAMP cascade stimulated by Cyr1),54 as well as serum and appears to be the principal filamentation regulator under most environmental conditions.45,48 Hyphal induction through Cph1 requires a mitogen-activated protein kinase (MAPK) pathway, which can be activated by low nitrogen levels. Moreover, both the cAMP and MAPK cascades are stimulated by Ras1, which further displays the complexity of the signaling circuitry.45 Czf1 stimulates filamentation via a signaling pathway involving Dck1 and is triggered by embedded and hypoxic conditions.55 Hgc1 is known to form a complex with Cdc28 and together they are able to induce hyphal growth by phosphorylating Efg1, leading to the repression of the cell separation machinery 56 and it has been proposed that Ume6 drives filamentation through this Hgc1 pathway.50 The GATA-family transcription factor Brg1 has also been shown to play a crucial role in filamentation.51 An overexpression strain of BRG1 was shown to filament under yeast growth conditions (YPD at 30°C) with a concomitant upregulation of hypha-specific genes such as ALS3, HWP1, and ECE1.51
Stimulation of the signaling transduction pathways described above leads to the induction of hypha-specific genes. Among these, ALS3, ECE1, and HWP1 which have recently been identified as part of a core filamentation response (CFR) network composed of 8 genes.57 This network also includes HGT2, IHD1, RBT1, DCK1, and the open reading frame orf19.2457, whose function is unknown. These CFR members were only upregulated in the early and late filamentation responses and not during the initial stage of germ tube formation.57 Using different media conditions, individual filamentation pathways can be stimulated and studied. Media such as Lee's is able to induce filamentation through the Cph2 and Tec1 pathway, Spider medium, which is carbon limiting, will induce filamentation through the cAMP pathway, while GlcNAc mediium is able to induce filamentation through Efg1.44
Filamentation modulates host immune responses during C. albicans infection
Cells of the immune system as well as epithelial and endothelial cells sense and respond to C. albicans yeast and filamentous cells in different ways, ultimately evoking distinct immune responses that can either help in the resolution of infection or contribute to pathology.3 Perhaps the best example of this is how mucosal epithelial cells are capable of discriminating between yeast cells and hyphae, with the former stimulating protective responses and the latter resulting in an overly exacerbated inflammatory response, which contributes to the pathology of both vaginal and oral candidiasis.58,59 Therefore, one additional advantage of an anti-virulence treatment that acts by inhibiting C. albicans filamentation would be the ensuing modulation of host immune responses in a manner that favors the host in fighting the infection. As such, under certain circumstances an anti-virulence strategy can be somewhat comparable to the use of live attenuated vaccines to induce protective immune responses.
The search for small molecule compounds with inhibitory activity against C. albicans filamentation
Although historically numerous molecules have been reported to exert an inhibitory effect on the C. albicans yeast to hypha transition, very few of these compounds were tested for their effects on virulence in vivo,60 and there were also some potential concerns about their overall toxicity. Thus, to date it has been difficult to translate these basic science discoveries into new therapies to combat C. albicans infections. In the last few years several groups have implemented more targeted efforts using high content phenotypic screens for the identification of small molecule inhibitors of C. albicans filamentation.
Screening for compounds that could inhibit the C. albicans yeast to hyphae transition in response to carbon limitation in Spider media, Toenjes et al. identified 5 novel small molecules and 16 molecules with known mammalian targets.61,62 These known compounds were inhibitors of protein kinases, protein phosphatases, Ras signaling pathways, G protein-coupled receptors, calcium homeostasis, nitric oxide and guanylate cyclase signaling, and apoptosis in mammalian cells. Interestingly several of these molecules were also capable of inhibiting filamentation in other Candida species and Aspergillus fumigatus, suggesting a somewhat broad spectrum of action. Since the original screen used the carbon-limiting hyphal-inducing signal of Spider media, it was implied that the identified compounds inhibited C. albicans filamentation by blocking the Efg1 pathway, although subsequent evaluation of their activity in different hyphal-inducing media indicated that some of the inhibitors may act through multiple signaling pathways.63 The group also performed chemical epistasis analyses to provide further insights into mechanism of action and potential molecular targets.63 However, none of these inhibitors were tested in infection models.
A high throughput phenotypic screening of 30,000 small molecules from a commercial library (DIVERset, Chembridge) in search for compounds that prevent adhesion of C. albicans to polystyrene surfaces identified several bioactive molecules.64 Besides attachment to plastic, several compounds also inhibited adhesion to epithelial cells, C. albicans morphogenesis and biofilm formation. The main leading compound was termed “filastatin” (see Fig. 2 for structure) based on its strong and long-lasting inhibition of filamentation.64 Filastatin blocks the transcriptional induction of the hyphal-specific HWP1 promoter and subsequent experiments indicated that filastatin acts downstream of multiple (but not all) signaling pathways; in particular filastatin blocks hyphal formation induced by serum, Spider media, and GlcNac, but not by the genotoxic stress agent hydroxyurea. Of note, filastatin exhibited antifungal activity in a nematode model of C. albicans infection and also in an ex vivo mouse model of vulvovaginal candidiasis.64
Figure 2.
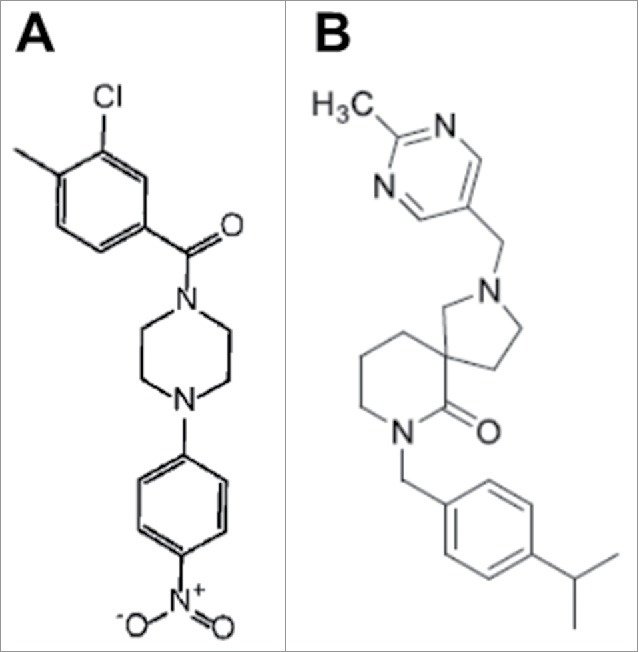
Chemical structures of filastatin (A) and compound 61894700 (B).
A cell-based phenotypic screen was performed using 3 different chemical libraries from the National Cancer Institute's Open Chemical Repository collection (Natural set, Structural Diversity set, and Challenge set), which identified several compounds with inhibitory activity against C. albicans filamentation.65 To screen for filamentation the authors took advantage of the tight control of morphogenetic conversions in the genetically engineered C. albicans tet-NRG1 strain,35 in order to develop an easy, inexpensive and robust screen for inhibitors of filamentation using a 96 well microtiter plate-based assay. Of the 2,293 compounds screened, there were a total of 17 confirmed hits identified as inhibitors of filamentation in C. albicans. The antibiotic activity of several of these compounds had already been previously described, but the screen also identified a few novel compounds. Nevertheless, most of the compounds identified as hits were cytotoxic, which severely limits their potential as candidates for the development of new antifungal drugs. Because of this reason, their in vivo efficacy was not further examined.
Most recently, Pierce et al. performed a large-scale, whole-cell assay screen of 20,000 small molecules from the research-intensive and medicinally relevant NOVACore™ chemical library (Chembridge) to identify compounds that inhibit C. albicans biofilm formation.66 The screen identified a novel hit series of diazaspiro-decane structural analogs, which was largely represented among the bioactive compounds and which is predicted to possess very favorable “drug-like” physical chemical properties. Not surprisingly, since filamentation plays a pivotal role in biofilm formation in this pathogenic fungus, an in depth characterization of the leading compound from this series (compound 61894700, Fig. 2) indicated that it also displays a potent inhibitory activity of C. albicans filamentation, and this activity was observed at relatively low concentrations at which the compound did not inhibit overall growth under planktonic conditions.66 Therefore, in contrast to conventional antifungals that target cell viability, this leading candidate seems to represent a true anti-virulence compound. Of note, serial passage experiments in vitro indicated that repeated exposure to the lead compound did not lead to the development of resistance, thereby confirming that this anti-virulence strategy is highly unlikely to foster the emergence of resistance.66 The anti-filamentation activity was retained in the presence of serum, which is essential from a drug development point of view. Also, the toxicity values of this leading compound determined in a standard assay using human hepatocytes were considerably higher than the effective concentrations against filamentation, presumably indicating a good safety profile. Thus, based on its promising in vitro characteristics a series of experiments were performed to assess its activity in vivo. Results of these experiments indicated that the leading compound exhibits activity in clinically-relevant murine models of both invasive and oral candidiasis and, consistent with its effect on fungal morphology and its anti-virulence mode of action, the efficacy of the treatment was associated with inhibition of filamentation in vivo as assessed by histological observations.66 In the invasive model, characteristic filamentous lesions were predominant in kidneys (the main target organ) from untreated mice, whereas isolated or small groups of mostly yeast cells were observed in kidney sections retrieved from mice treated with the leading compound. Similarly in the oral model, the tongues from untreated mice demonstrated a widespread biofilm and extensive lesions with numerous hyphae penetrating and causing damage to the epithelium, as opposed to superficially located scattered yeast cells in tongues from mice treated with this compound.66 Importantly, the model of oral candidiasis uses immunosuppressed mice;67,68 thereby these results would suggest that an anti-virulence strategy could potentially be used to treat certain forms of candidiasis, even in immune deficient patients.
Conclusions and outlook
Infections caused by C. albicans continue to represent a major challenge to an expanding population of at-risk patients. These infections carry unacceptably high morbidity and mortality rates, which clearly points to major limitations in our current antifungal armamentarium. However, the development of novel antifungal drugs is difficult due to the scarcity of specific fungal targets. The increased knowledge on C. albicans pathogenesis acquired through years of arduous work by multiple groups of investigators, particularly in the last 10–15 y after the completion of the initial C. albicans genome project, provides for an unprecedented opportunity and framework for the discovery and development of novel antifungal drugs. Thus, two main questions remain. First, will we be able to harness this knowledge and apply these insights from C. albicans biology and pathogenicity in order to develop a new approach for the treatment of these infections? And second, when will this investment pay off for antifungal drug discovery?
These anti-virulence therapeutics will shift the advantage to the host by effectively devoiding this fungus of its pathogenic potential, and they will be less likely to foster the emergence of resistance, thereby expanding the number of potential targets and constituting a new paradigm for the development of drugs with novel modes of action. Another of the major advantages of an anti-virulence strategy is the maintenance of the microbiome and the normal yeast ecology, as suppression of C. albicans populations due to antifungal treatment contributes to the changing epidemiology of candidiasis, with the substitution of C. albicans by species that are intrinsically less susceptible to conventional antifungals (i.e., C. krusei, C. glabrata). Thus, even in the absence of direct “activity” against non-albicans species, it is entirely possible that the preservation of a commensal state with C. albicans while under treatment with an anti-filamentation compound will suppress proliferation of these other Candida species. An important caveat is that the successful implementation of anti-virulence approaches will also require the rapid and accurate diagnosis of C. albicans infections, which also remains a major challenge. Taken together, as the best studied virulence factor in C. albicans, filamentation represents an attractive target, already validated at the genetic level, for the development of anti-virulence approaches against candidiasis. This accumulated information, together with the implementation of high throughput/high content screenings in search for specific inhibitors of C. albicans filamentation, stand poised to deliver much needed novel compounds to the antifungal development pipeline.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Work in the laboratory is supported by NIH grants R01DE023510 and R01AI119554 from the National Institute of Dental and Craneofacial Research and the National Institute of Allergy and Infectious Diseases, respectively to JLLR. Additional support is provided by the Army Research Office of the Department of Defense under Contract No. W911NF-11-1-0136 (to JLLR and SPS) and by the Margaret Batts Tobin Foundation, San Antonio, TX. JAR is supported by the UTSA RISE-PhD Trainee Program (NIH/NIGMS RISE GM60655). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, and the content is solely the responsibility of the authors.
References
- [1].Calderone RA. Candida and Candidiasis. ASM Press: Washington: 2002. [Google Scholar]
- [2].Kadosh D, Lopez-Ribot JL. Candida albicans: adapting to succeed. Cell Host Microbe 2013; 14:483-5; PMID:24237692; http://dx.doi.org/ 10.1016/j.chom.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Romani L, Bistoni F, Puccetti P. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr Opin Microbiol 2003; 6:338-43; PMID:12941401; http://dx.doi.org/ 10.1016/S1369-5274(03)00081-X [DOI] [PubMed] [Google Scholar]
- [4].Salvatori O, Puri S, Tati S, Edgerton M. Innate Immunity and Saliva in Candida albicans-mediated Oral Diseases. J Dent Res 2016; 95:365-71; PMID:26747422; http://dx.doi.org/ 10.1177/0022034515625222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13; PMID:23253612; http://dx.doi.org/ 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- [6].Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133-63; PMID:17223626; http://dx.doi.org/ 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McCarty TP, Pappas PG. Invasive Candidiasis. Infect Dis Clin North Am 2016; 30:103-24; PMID:26739610; http://dx.doi.org/ 10.1016/j.idc.2015.10.013 [DOI] [PubMed] [Google Scholar]
- [8].Lopez-Martinez R. Candidosis, a new challenge. Clin Dermatol 2010; 28:178-84; PMID:20347660; http://dx.doi.org/ 10.1016/j.clindermatol.2009.12.014 [DOI] [PubMed] [Google Scholar]
- [9].Redding SW, Zellars RC, Kirkpatrick WR, McAtee RK, Caceres MA, Fothergill AW, Lopez-Ribot JL, Bailey CW, Rinaldi MG, Patterson TF. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol 1999; 37:3896-900; PMID:10565903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thompson GR 3rd, Patel PK, Kirkpatrick WR, Westbrook SD, Berg D, Erlandsen J, Redding SW, Patterson TF. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109:488-95; PMID:20156694; http://dx.doi.org/ 10.1016/j.tripleo.2009.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sobel JD. Vulvovaginal candidosis. Lancet (London, England) 2007; 369:1961-71; PMID:17560449; http://dx.doi.org/ 10.1016/S0140-6736(07)60917-9 [DOI] [PubMed] [Google Scholar]
- [12].Kullberg BJ, Arendrup MC. Invasive Candidiasis. The New England journal of medicine 2015; 373:1445-56; PMID:26444731; http://dx.doi.org/ 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- [13].Grubb SE, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect Immun 2008; 76:4370-7; PMID:18573891; http://dx.doi.org/ 10.1128/IAI.00332-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol 2003; 11:272-9; PMID:12823944; http://dx.doi.org/ 10.1016/S0966-842X(03)00117-3 [DOI] [PubMed] [Google Scholar]
- [15].Pierce CG, Srinivasan A, Uppuluri P, Ramasubramanian AK, López-Ribot JL. Antifungal therapy with an emphasis on biofilms. Curr Opin Pharmacol 2013; 13:726-30; PMID:24011516; http://dx.doi.org/ 10.1016/j.coph.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].te Welscher YM, van Leeuwen MR, de Kruijff B, Dijksterhuis J, Breukink E. Polyene antibiotic that inhibits membrane transport proteins. Proc Natl Acad Sci U S A 2012; 109:11156-9; PMID:22733749; http://dx.doi.org/ 10.1073/pnas.1203375109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, et al.. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 2014; 10:400-6; PMID:24681535; http://dx.doi.org/ 10.1038/nchembio.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 2012; 109:2234-9; PMID:22308411; http://dx.doi.org/ 10.1073/pnas.1117280109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 2010; 9:719-27; PMID:20725094; http://dx.doi.org/ 10.1038/nrd3074 [DOI] [PubMed] [Google Scholar]
- [20].Perea S, Lopez-Ribot JL, Kirkpatrick WR, McAtee RK, Santillan RA, Martinez M, Calabrese D, Sanglard D, Patterson TF. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2001; 45:2676-84; PMID:11557454; http://dx.doi.org/ 10.1128/AAC.45.10.2676-2684.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Current opinion in infectious diseases 2014; 27:484-92; PMID:25304391; http://dx.doi.org/ 10.1097/QCO.0000000000000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hernandez S, Lopez-Ribot JL, Najvar LK, McCarthy DI, Bocanegra R, Graybill JR. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother 2004; 48:1382-3; PMID:15047549; http://dx.doi.org/ 10.1128/AAC.48.4.1382-1383.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wiederhold NP, Grabinski JL, Garcia-Effron G, Perlin DS, Lee SA. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob Agents Chemother 2008; 52:4145-8; PMID:18794385; http://dx.doi.org/ 10.1128/AAC.00959-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Denning DW, Bromley MJ. Infectious Disease. How to bolster the antifungal pipeline. Science (New York, N Y ) 2015; 347:1414-6; PMID:25814567; http://dx.doi.org/ 10.1126/science.aaa6097 [DOI] [PubMed] [Google Scholar]
- [25].Casadevall A, Pirofski LA. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun 1999; 67:3703-13; PMID:10417127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Casadevall A, Pirofski LA. Microbial virulence results from the interaction between host and microorganism. Trends Microbiol 2003; 11:157-8; author reply 8-9; PMID:12706990; http://dx.doi.org/ 10.1016/S0966-842X(03)00008-8 [DOI] [PubMed] [Google Scholar]
- [27].Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence 2013; 4:119-28; PMID:23302789; http://dx.doi.org/ 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 2008; 6:17-27; PMID:18079741; http://dx.doi.org/ 10.1038/nrmicro1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 2007; 3:541-8; PMID:17710100; http://dx.doi.org/ 10.1038/nchembio.2007.24 [DOI] [PubMed] [Google Scholar]
- [30].Chaffin WL, Lopez-Ribot JL, Casanova M, Gozalbo D, Martinez JP. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev 1998; 62:130-80; PMID:9529890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol 1991; 45:187-218; PMID:1741614; http://dx.doi.org/ 10.1146/annurev.mi.45.100191.001155 [DOI] [PubMed] [Google Scholar]
- [32].Calderone RA, Cihlar RL. Fungal pathogenesis Principles and Clinical Applications. Marcel Decker: New York: 2002. [Google Scholar]
- [33].MacCallum DM, Odds FC. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 2005; 48:151-61; PMID:15842329; http://dx.doi.org/ 10.1111/j.1439-0507.2005.01121.x [DOI] [PubMed] [Google Scholar]
- [34].Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun 2000; 68:3485-90; PMID:10816502; http://dx.doi.org/ 10.1128/IAI.68.6.3485-3490.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2003; 2:1053-60; PMID:14555488; http://dx.doi.org/ 10.1128/EC.2.5.1053-1060.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell 1997; 90:939-49; PMID:9298905; http://dx.doi.org/ 10.1016/S0092-8674(00)80358-X [DOI] [PubMed] [Google Scholar]
- [37].Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, Lopez-Ribot JL. Use of a genetically engineered strain to evaluate the pathogenic potential of yeast cell and filamentous forms during Candida albicans systemic infection in immunodeficient mice. Infect Immun 2008; 76:97-102; PMID:17967861; http://dx.doi.org/ 10.1128/IAI.00982-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Saville SP, Lazzell AL, Bryant AP, Fretzen A, Monreal A, Solberg EO, Monteagudo C, Lopez-Ribot JL, Milne GT. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob Agents Chemother 2006; 50:3312-6; PMID:17005810; http://dx.doi.org/ 10.1128/AAC.00628-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol 2001; 9:327-35; PMID:11435107; http://dx.doi.org/ 10.1016/S0966-842X(01)02094-7 [DOI] [PubMed] [Google Scholar]
- [40].Odds FC, Gow NA, Brown AJ. Fungal virulence studies come of age. Genome Biol 2001; 2:1009.1-4; PMID:11276429; http://dx.doi.org/ 10.1186/gb-2001-2-3-reviews1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Soll DR. Candida commensalism and virulence: the evolution of phenotypic plasticity. Acta Trop 2002; 81:101-10; PMID:11801217; http://dx.doi.org/ 10.1016/S0001-706X(01)00200-5 [DOI] [PubMed] [Google Scholar]
- [42].Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J 2004; 23:1845-56; PMID:15071502; http://dx.doi.org/ 10.1038/sj.emboj.7600195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 2010; 42:590-8; PMID:20543849; http://dx.doi.org/ 10.1038/ng.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Berman J, Sudbery PE. Candida Albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet 2002; 3:918-30; PMID:12459722; http://dx.doi.org/ 10.1038/nrg948 [DOI] [PubMed] [Google Scholar]
- [45].Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol 2011; 9:737-48; PMID:21844880; http://dx.doi.org/ 10.1038/nrmicro2636 [DOI] [PubMed] [Google Scholar]
- [46].Alves CT, Silva S, Pereira L, Williams DW, Azeredo J, Henriques M. Effect of progesterone on Candida albicans vaginal pathogenicity. Int J Med Microbiol 2014; 304:1011-7; PMID:25183575; http://dx.doi.org/ 10.1016/j.ijmm.2014.07.004 [DOI] [PubMed] [Google Scholar]
- [47].Kadosh D. Shaping up for battle: morphological control mechanisms in human fungal pathogens. PLoS Pathog 2013; 9:e1003795; PMID:24385899; http://dx.doi.org/ 10.1371/journal.ppat.1003795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in candida albicans. Genetics 2000; 155:57-67; PMID:10790384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C.albicans, is down-regulated during filament induction. Embo J 2001; 20:4753-61; PMID:11532939; http://dx.doi.org/ 10.1093/emboj/20.17.4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci U S A 2009; 106:599-604; PMID:19116272; http://dx.doi.org/ 10.1073/pnas.0804061106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cleary IA, Lazzell AL, Monteagudo C, Thomas DP, Saville SP. BRG1 and NRG1 form a novel feedback circuit regulating Candida albicans hypha formation and virulence. Mol Microbiol 2012; 85:557-73; PMID:22757963; http://dx.doi.org/ 10.1111/j.1365-2958.2012.08127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 1994; 266:1723-6; PMID:7992058; http://dx.doi.org/ 10.1126/science.7992058 [DOI] [PubMed] [Google Scholar]
- [53].Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. Embo J 1997; 16:1982-91; PMID:9155024; http://dx.doi.org/ 10.1093/emboj/16.8.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell 2001; 12:3631-43; PMID:11694594; http://dx.doi.org/ 10.1091/mbc.12.11.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Brown DH Jr., Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol 1999; 34:651-62; PMID:10564506; http://dx.doi.org/ 10.1046/j.1365-2958.1999.01619.x [DOI] [PubMed] [Google Scholar]
- [56].Wang A, Raniga PP, Lane S, Lu Y, Liu H. Hyphal chain formation in Candida albicans: Cdc28-Hgc1 phosphorylation of Efg1 represses cell separation genes. Mol Cell Biol 2009; 29:4406-16; PMID:19528234; http://dx.doi.org/ 10.1128/MCB.01502-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Martin R, Albrecht-Eckardt D, Brunke S, Hube B, Hunniger K, Kurzai O. A core filamentation response network in Candida albicans is restricted to eight genes. PloS One 2013; 8:e58613; PMID:23516516; http://dx.doi.org/ 10.1371/journal.pone.0058613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fidel PL Jr., Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 2004; 72:2939-46; PMID:15102806; http://dx.doi.org/ 10.1128/IAI.72.5.2939-2946.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, et al.. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010; 8:225-35; PMID:20833374; http://dx.doi.org/ 10.1016/j.chom.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shareck J, Belhumeur P. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryot Cell 2011; 3:3; PMID:NOT_FOUND [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Toenjes KA, Munsee SM, Ibrahim AS, Jeffrey R, Edwards JE Jr., Johnson DI. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob Agents Chemother 2005; 49:963-72; PMID:15728890; http://dx.doi.org/ 10.1128/AAC.49.3.963-972.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Toenjes KA, Stark BC, Brooks KM, Johnson DI. Inhibitors of cellular signalling are cytotoxic or block the budded-to-hyphal transition in the pathogenic yeast Candida albicans. J Med Microbiol 2009; 58:779-90; PMID:19429755; http://dx.doi.org/ 10.1099/jmm.0.006841-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Midkiff J, Borochoff-Porte N, White D, Johnson DI. Small molecule inhibitors of the Candida albicans budded-to-hyphal transition act through multiple signaling pathways. PLoS One 2011; 6:e25395; PMID:21966518; http://dx.doi.org/ 10.1371/journal.pone.0025395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fazly A, Jain C, Dehner AC, Issi L, Lilly EA, Ali A, Cao H, Fidel PL Jr., Rao RP, Kaufman PD. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc Natl Acad Sci U S A 2013; 110:13594-9; PMID:23904484; http://dx.doi.org/ 10.1073/pnas.1305982110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pierce CG, Saville SP, Lopez-Ribot JL. High-content phenotypic screenings to identify inhibitors of Candida albicans biofilm formation and filamentation. Pathog Dis 2014; 70:423-31; PMID:24623598; http://dx.doi.org/ 10.1111/2049-632X.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pierce CG, Chaturvedi AK, Lazzell AL, Powell AT, Saville SP, McHardy SF, Lopez-Ribot JL. A novel small molecule inhibitor of biofilm formation, filamentation and virulence with low potential for the development of resistance. NPJ Biofilms Microbiomes 2015; 1:15012; PMID:26691764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Solis NV, Filler SG. Mouse model of oropharyngeal candidiasis. Nat Protoc 2012; 7:637-42; PMID:22402633; http://dx.doi.org/ 10.1038/nprot.2012.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, Abe S. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol 2003; 47:321-6; PMID:12825893; http://dx.doi.org/ 10.1111/j.1348-0421.2003.tb03403.x [DOI] [PubMed] [Google Scholar]


