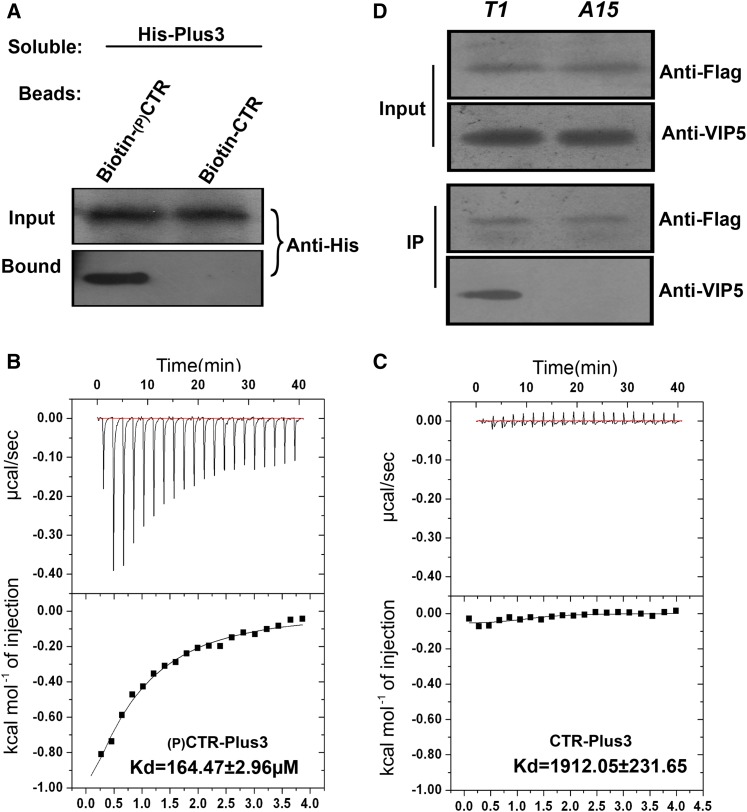
Figure 6.
Interaction between SPT5 and VIP5 Depends on CTR Phosphorylation.
(A) Binding of soluble His-Plus to bead-bound peptides containing three complete CTR repeats was measured. The 18-mer peptides were either nonphosphorylated (Non-P) or phosphorylated at threonine. The amount of His-VIP5 protein bound to the peptides on the beads was determined by an immunoblot analysis with antibody to His (Anti-His).
(B) and (C) Measurement of phosphorylated or nonphosphorylated peptides described as binding to the Plus3 domain of VIP5 (A) using isothermal titration calorimetry at 25°C. A 200-μL sample of 150 μM Plus3 was titrated with 2 μL injections of 3 mM phosphorylated peptides (B) or nonphosphorylated peptide (C) at 120-s intervals. Curves are the best fits to the data that enable the acquisition of the stoichiometry and thermodynamic parameters. Experiments were repeated at least three times, and a representative experiment is shown.
(D) Coimmunoprecipitation of the wild-type or nonphosphorylatable form of SPT5 with VIP5. The cell extractions from the SPT5-complemented (T1) or nonphosphorylatable SPT5-complemented spt5 seedling (A15) were immunoprecipitated with an anti-FLAG antibody and then detected with anti-VIP5 antibody.