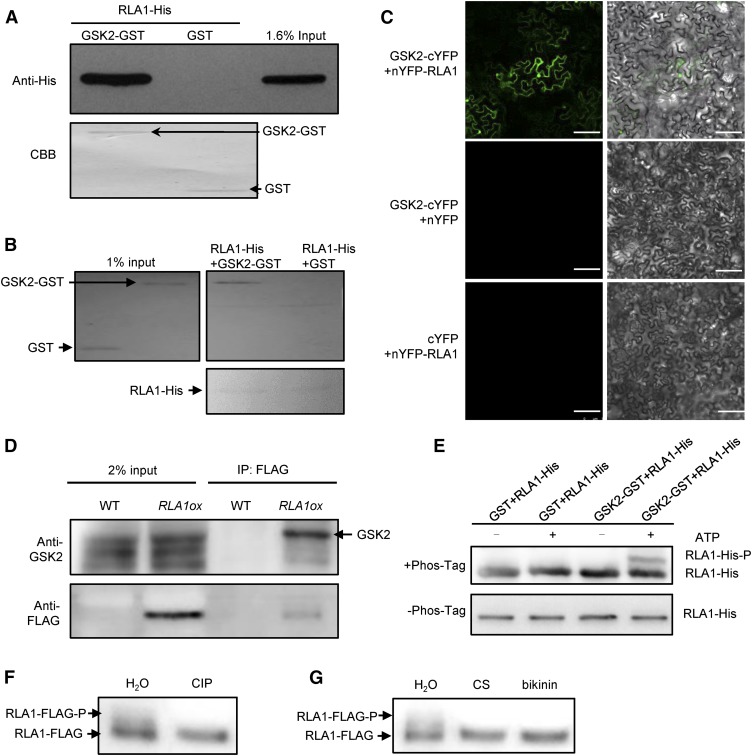
Figure 7.
GSK2 Interacts with and Phosphorylates RLA1 in Vitro and in Vivo.
(A) GSK2-GST proteins can pull down RLA1-His proteins by GST pull-down assays in vitro. GSK2-GST and GST were stained with Ponceau S as loading controls.
(B) RLA1-His proteins can pull down GSK2-GST proteins in vitro. RLA1-His proteins were stained with Ponceau S as loading controls.
(C) Interaction between RLA1 and GSK2 in BiFC assays. Bars = 100 μm.
(D) Interaction between RLA1 and GSK2 in co-IP assays. The proteins were extracted from wild-type or RLA1ox plants and immunoprecipitated by Anti-FLAG magnetic beads. Gel blots were probed with anti-FLAG or anti-GSK2 antibody.
(E) The in vitro kinase assay of RLA1 by GSK2 kinase using a Phos-tag gel. Proteins were detected by immunoblotting with anti-His antibodies. An equal amount of each recombinant protein was separated on the gel without the Phos-tag as a loading control.
(F) Immunoprecipitated RLA1-FLAG protein from RLA1ox plants was treated with CIP or water. The signal was detected by anti-FLAG.
(G) Immunoprecipitated RLA1-FLAG protein from the RLA1ox plants grown on medium containing 100 nM CS or 50 μM bikinin. The signal was detected by anti-FLAG.